Press release
OD-OS earns FDA Approval for its NAVILAS navigated laser therapy system
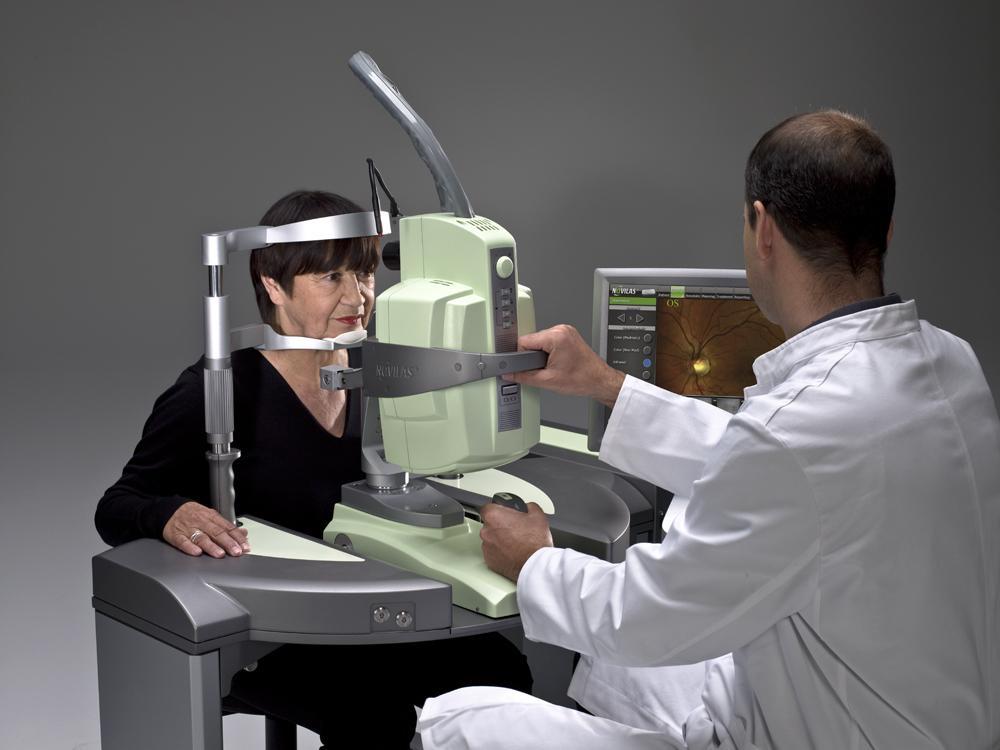
NAVILAS® Laser System Earns FDA Approval(Dallas, Texas, Nov 2, 2009) OD-OS announces that it has earned FDA 510k Clearance to market NAVILAS®, its product for retina laser therapy. The new navigated laser system incorporates advanced imaging with retinal laser therapy in a planned and controlled way using image registration in one device with integrated workflow to improve the safety, accuracy, speed, comfort and transparency of retina laser photocoagulation. Laser photocoagulation is the current standard in the treatment of many retinal disorders including Diabetic Retinopathy and Diabetic Macular Edema (DME).
NAVILAS®, allows physicians to move beyond conventional slit lamp based photocoagulation that provides only a partial slit image of the retina. The NAVILAS® system produces a live image of the fundus in true color, IR and red-free as well as fluorescien angiography (FA) with 50 degree field of view on a monitor – panable across the fundus. In contrast to fundus cameras or scanning laser ophthalmoscopes, this patented imaging technology is capable of real-time imaging of the retina in color – both mydratic and non-mydratic - while including a treatment laser to treat the retina.
“In trials, initial accuracy results with NAVILAS® indicate a microaneurysm hit rate of 92% as evidenced by post treatment color overlay photographs,” reported William Freeman, M.D., Professor and Director of the Jacobs Retina Center at the UCSD Shiley Eye Center, in La Jolla, CA, during his presentation at Retina Subspecialty Day of the AAO 2009 in San Francisco..
With NAVILAS®, doctors have freedom of neck movement and are able to adjust their height and proximity to the patient for maximum ergonomic benefits. “The neck and back strain of slit lamp examination will be greatly reduced,” said Dr. Michael Ober, Retina Consultants of Michigan, Southfield Michigan.
NAVILAS® made its first appearances in the US in New York in September at the Retina Congress and last week at the American Academy of Ophthalmology in San Francisco. “In both venues, doctors were excited by the new technology that will, for the first time, allow them to use what we call Retina Navigation, the ability to IMAGE – PLAN – TREAT and DOCUMENT retina disorders,“ said Winfried Teiwes, CEO.
For more information on the NAVILAS® system, call (877) 628-6367 or visit www.od-os.com.
About OD-OS
OD-OS is an ISO 9001 and ISO 13485 certified medical device company headquartered in Teltow/Berlin, Germany. Its US operation, responsible for marketing, sales and support, is located Grapevine/Dallas, Texas.
OD-OS is a subsidiary of SensoMotoric Instruments (SMI), the technology leader in eye tracking and registration solutions for refractive laser surgery. Over the past 15 years, SMI has supplied technology to most major refractive laser manufacturers.
In 2008, after more than 4 years of development creating Retina Navigation within SMI, the core development team was spun-out into OD-OS and charged with resources and ophthalmic industry experts to bring Retina Navigation with its first product, NAVILAS, to the market. For more information visit www.od-os.com or in the US call 1 (877) 628-6367 or Europe at +49 (3328) 31 282-100.
# # #
Photos and interviews available upon request
OD-OS GmbH
Warthestrasse 21
D-14513 Teltow
www.OD-OS.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release OD-OS earns FDA Approval for its NAVILAS navigated laser therapy system here
News-ID: 105535 • Views: …
More Releases for NAVILAS
Retinal Laser Photocoagulation Market Dynamics Fueled by Innovation in Argon Gre …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Retinal Laser Photocoagulation Market Size, Share & Trends Analysis Report By Laser Type ((Argon Laser (488 nm), Green Laser (532 nm), Yellow Laser (577 nm), Diode Laser (810-1050 nm)}, By Indication (Proliferative Diabetic Retinopathy (PDR), Diabetic Macular Edema (DME), Retinal Vein Occlusion (RVO), Retinal Tears, Holes and Lattice Degeneration, Central Serous Chorioretinopathy (CSC), Choroidal Neovascularization…
United States Cocoa Nibs Market: Organic Sourcing, Traceability & Ethical Growth …
The Global Cocoa Nibs Market is expected to reach at a significant CAGR during the forecast period (2025-2032).
DataM Intelligence has published a new research report on "Cocoa Nibs Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current…
Retinal Laser Photocoagulation Market Top Companies Study - Alcon, A.R.C Laser, …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Retinal Laser Photocoagulation Market Size, Share & Trends Analysis Report By Laser Type ((Argon Laser (488 nm), Green Laser (532 nm), Yellow Laser (577 nm), Diode Laser (810-1050 nm)}, By Indication (Proliferative Diabetic Retinopathy (PDR), Diabetic Macular Edema (DME), Retinal Vein Occlusion (RVO), Retinal Tears, Holes and Lattice Degeneration, Central Serous Chorioretinopathy (CSC), Choroidal Neovascularization…
Ophthalmic Lasers Market Set to Surge to $1,909.7 Million by 2030 at a 4.7% CAGR
The global ophthalmic lasers market has witnessed significant growth in recent years, driven by technological advancements and an increasing prevalence of eye-related diseases. In 2020, the ophthalmic lasers market was valued at approximately $1,207.81 million and is projected to reach $1,909.71 million by 2030, registering a CAGR of 4.7% from 2021 to 2030.
Read More: https://www.alliedmarketresearch.com/ophthalmic-laser-market
Ophthalmic Lasers Market Growth Factors
Several factors contribute to the growth of the ophthalmic lasers market:
-…
Retinal Laser Photocoagulation Market Top Players - Alcon, A.R.C Laser, Ellex Me …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Retinal Laser Photocoagulation Market Size, Share & Trends Analysis Report By Laser Type ((Argon Laser (488 nm), Green Laser (532 nm), Yellow Laser (577 nm), Diode Laser (810-1050 nm)}, By Indication (Proliferative Diabetic Retinopathy (PDR), Diabetic Macular Edema (DME), Retinal Vein Occlusion (RVO), Retinal Tears, Holes and Lattice Degeneration, Central Serous Chorioretinopathy (CSC), Choroidal Neovascularization…
Cocoa Nibs Market Growth Surge: Key Players and Market Insights-Barry Callebaut …
DataM Intelligence has released a new research report on the Cocoa Nibs market. The report provides a detailed analysis of current and emerging trends, offering insights into the market dynamics. It utilizes Porter's Five Forces model to assess key factors such as supplier and customer relationships, risks from various agents, competitive intensity, and opportunities for new entrants. The study also includes research data from various companies, analyzing factors like benefits,…