Press release
Regulatory Information Management Market Projected to Garner Significant Revenues by 2026
The technological advancements and novel methods in the regulatory information management has unlocked potential for the companies, which are striving to provide regulatory information management software. The regulatory information management software has developed with new technologies, and improved process to ensure effective management of regulatory information. The pharmaceutical companies involved in the development, dissemination, capture, and control the regulatory information through the product development cycle. Regulatory information management is a solution for pharmaceutical companies in terms of efficient value communication of regulations. The technology tools, product & service platforms, creates the opportunities for the automating business practices. RIM is enables deliver powerful submission of planning, viewing, publishing, registration and management of products throughout the life cycle. RIMS allows ensure effective, compliant management of regulations and regulatory information. It has advantages such as submission of plan, e-submission viewer, and product registration and tracking. It enables track the periodic safety updated reports (PSUR) and real time access of regulatory information. Global Regulatory Information Management Market is anticipated to exhibit a significant CAGR coupled with annual growth rates over the forecast period.A sample of this report is available upon request @ https://www.futuremarketinsights.com/reports/sample/rep-gb-1889
Regulatory Information Management Market: Drivers and restraints
Regulatory Information Management Market provides wide range of benefits to pharmaceutical companies, increased amount of data to be collected and provided by regulatory bodies such as U.S. Food and Drug Administration (FDA), European Medicines Agency. According to the EVMPD regulation, all pharmaceutical companies harmonized exchanged format to manage the drug safety product information, which effected on July 02, 2012. The regulatory bodies are majorly fuelling the global regulatory information management market over the forecast period. Pharmaceutical companies are always fond of advanced technologies such as regulatory information management system, which enables easy access of information any time possible, thus most of the pharma companies are inclined towards the regulatory information management market. The reduction in manual errors in regulatory process coupled with easy access are further expected to contribute in the growth of global regulatory information management market
However, there are some disadvantages in the use of Regulatory Information Management system that includes the need to train the sales professionals to manage advanced technologies. Also Regulatory Information Management is costlier than conventional method. These factors can act as major road blocks for the growth Regulatory Information Management Market.
Regulatory Information Management Market: Segmentation
Global Regulatory Information Management Market can be segmented as following types
By Product:
Software
Hardware
By End User
Pharmaceutical Industry
Biotechnology Industry
Clinical Research organizations
Regulatory Information Management Market: Overview
The demand of Regulatory Information Management Market is increasing in pharmaceutical and biopharmaceutical companies due to its efficiency and effectiveness in regulatory approval of pharmaceuticals. Through regulatory information management market companies can share their product updates. The global Regulatory Information Management Market is expected to unlock the potential of market over the forecast period.
Regulatory Information Management Market: Region-Wise Outlook
Regulatory Information Management Market is segmented into seven key regions: Those are North America, Latin America, Eastern Europe, Western Europe, and APEJ, Japan, Middle East and Africa. North America is anticipated to dominate the regulatory information management market due to the presence of pharmaceutical stalwarts in the countries like US and Canada. North America is followed by Asia Pacific in terms of market share of regulatory information management market owing to the rise of pharmaceuticals industry in the countries like China and India. Europe is growing at a modest CAGR in the global regulatory information management market. Latin America and Middle East and Africa are at a nascent stage in the global regulatory information management market and is expected to have a significant contribution in the market in the forecast period.
To view TOC of this report is available upon request @ https://www.futuremarketinsights.com/toc/rep-gb-1889
Regulatory Information Management Market: Key players
Some of the key players are
ACUTA, LLC.
Parexel
Computer Sciences Corp (CSC)
Aris Global
Virtify
Ennov
Amplexor
Samarind Ltd
Dovel Technologies, Inc.
Informa
About Us
Future Market Insights is the premier provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in London, the global financial capital, and has delivery centres in the U.S. and India.
FMI’s research and consulting services help businesses around the globe navigate the challenges in a rapidly evolving marketplace with confidence and clarity. Our customised and syndicated market research reports deliver actionable insights that drive sustainable growth. We continuously track emerging trends and events in a broad range of end industries to ensure our clients prepare for the evolving needs of their consumers.
Contact Us:
Future Market Insights
616 Corporate Way,
Suite 2-9018,
Valley Cottage,
New York 10989,
United States
Tel: +1-347-918-3531
Fax: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Information Management Market Projected to Garner Significant Revenues by 2026 here
News-ID: 1037734 • Views: …
More Releases from Future Market Insights
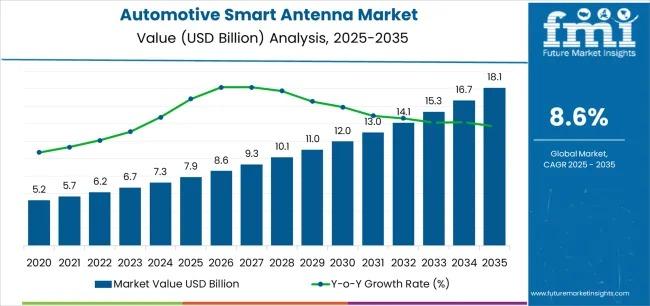
Global Automotive Smart Antenna Market to Reach USD 18.1 Billion by 2035, Driven …
The global automotive smart antenna market is projected to grow from USD 7.9 billion in 2025 to USD 18.1 billion by 2035, registering a robust compound annual growth rate (CAGR) of 8.6% during the forecast period. This expansion reflects the accelerating transformation of the automotive sector toward connected mobility, advanced communication infrastructure, and data-driven vehicle ecosystems. Increasing adoption of 5G-enabled connectivity, advanced driver assistance systems (ADAS), and vehicle-to-everything (V2X) communication…
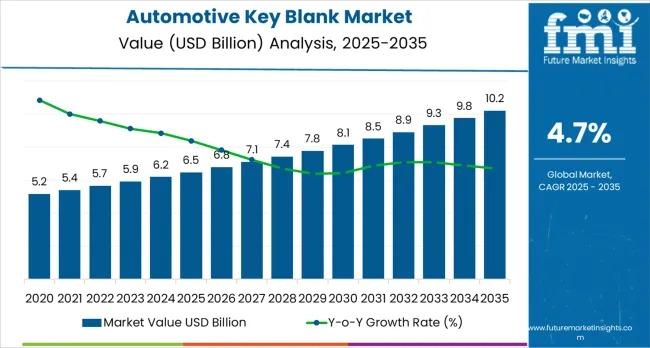
Automotive Key Blank Market to Reach USD 10.2 Billion by 2035 as Smart Key Integ …
The global automotive key blank market is projected to grow from USD 6.5 billion in 2025 to USD 10.2 billion by 2035, reflecting a steady compound annual growth rate (CAGR) of 4.7%. This expansion is being driven by rising vehicle production, increasing adoption of smart and transponder-based security systems, and sustained replacement demand across global automotive fleets. As vehicle access systems evolve toward integrated electronic security frameworks, automotive key blanks…
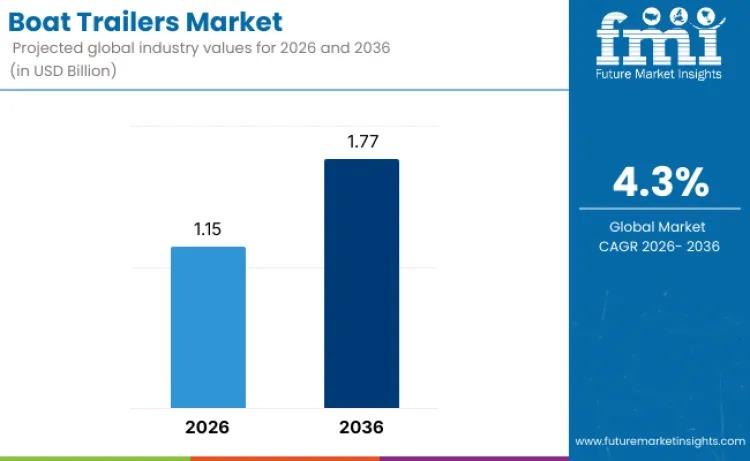
Global Boat Trailers Market to Reach USD 1.8 Billion by 2036 as Recreational Boa …
The global boat trailers market is poised for steady and sustained growth, with industry valuation projected to increase from USD 1.1 billion in 2026 to USD 1.8 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.3%. This expansion reflects rising global participation in recreational boating, increasing disposable incomes, and expanding investments in marine tourism infrastructure. As water-based leisure activities gain popularity across developed and emerging economies,…
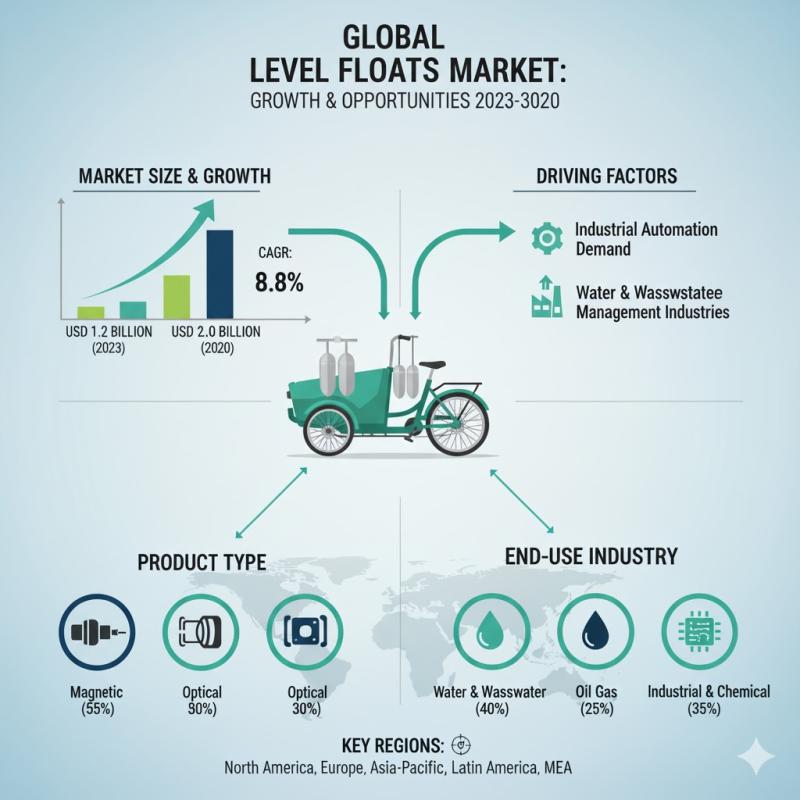
Level Floats Market to Reach USD 461.7 Million by 2035 as Automotive Electrifica …
The global level floats market is projected to grow from USD 311.9 million in 2025 to USD 461.7 million by 2035, registering a steady compound annual growth rate (CAGR) of 4.0% over the forecast period. This expansion reflects rising demand for precision fuel measurement systems across automotive, industrial, and energy applications, supported by increasing vehicle production, evolving fuel management technologies, and stricter regulatory standards focused on efficiency and emissions compliance.
Level…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
