Press release
CELL LINE DEVELOPMENT SERVICES MARKET TO WITNESS STEADY GROWTH IN THE COMING DECADE
Roots Analysis has announced the addition of “Cell Line Development Services Market, 2017-2027” report to its list of offerings. The report features an extensive study on the rapidly growing market of cell line development and manufacturing services providers. The report presents an elaborate compilation of research, analysis and opinions on several key aspects of the market.Akanksha Bhagtani, the principal analyst, said, “The growing pipeline of biological drugs has resulted in a continuous increase in the demand for different types of cell lines. Additionally, the loss of patent exclusivity of several biologics and the entry of numerous biosimilars, and need for cell lines in other areas, such as drug screening, gene functional studies, cell-based assay development, diagnostics and tissue engineering, have resulted in further increase in the demand for different types of cell lines. This presents significant opportunities to cell line development service providers.”
One of the primary objectives of the study was to project the growth of this market segment and evaluate the future prospects of upcoming cell line development technologies / services. Amongst other things, the study highlights the following:
• The current competitive market landscape with respect to key players, along with information on the location of their manufacturing facilities, distribution of cell lines based on their source of origin (mammalian, microbial, insect-derived, avian, marine and reptilian), type of cell lines (recombinant, hybridoma and primary cell lines), affiliated production technologies, purpose of production (R&D, diagnostics, biomanufacturing and tissue engineering), and other associated services (cell line characterization, cell banking, cell bank characterization, process development, cGMP manufacturing of biologics, fill / finish of end products and commercialization of reagents / equipments).
• A detailed analysis of the database presented as three schematic representations; a world map depicting the most active geographies in terms of the presence of cell line development facilities, a heat map representing the distribution of companies on the basis of their location (continent-wise distribution), year of establishment and cell line development capabilities, and a logo landscape highlighting the distribution of companies based on the number of employees and the source of cell lines.
• Elaborate profiles of key industry players that have proprietary technologies for the development of cell lines and offer other services, such as cell line characterization, cell banking and cGMP production of biologics, as well. Each profile features an overview of the company, its financial performance, information on cell line development services and proprietary technology, manufacturing facilities, expansions and collaborations, and a comprehensive future outlook.
• Detailed profiles of non-industry players (cell line repositories) that play an active role in the development of cell lines and offer affiliated services, as well. Each profile features an overview of the repository and a brief description of the cell line development services offered.
• An analysis of the future growth opportunity, segmented by regions, source and grade of cell lines. For the purposes of this analysis, several parameters, such as the number of companies involved, price of cell lines depending (characterized or uncharacterized), source of cell lines, and average number of cell line development projects undertaken by companies in a year, were taken into consideration.
Bhagtani further added, “Among different types of sources used to obtain cell lines, mammalian sources continue to be preferred (~80%); this is followed by microbial cell lines. Overall, the cell line development services market is poised to grow at a steady pace in the coming decade.”
The report highlights the contributions of several players in the field, including those that are listed below:
• Abzena
• Batavia Biosciences
• Celonic
• CMC Biologics
• Cobra Biologics
• Hyprocell
• LFB Biomanufacturing
• Lonza
• ProBioGen
• Selexis
• trenzyme
The opinions and insights discussed in this report were influenced by discussions conducted with industry experts. The report features detailed transcripts of interviews held with Fan Chen (Vice President Bioprocessing, LakePharma), Michael Pointek (Managing Director, Artes Biotechnology), Nienke Smits (Business Development, Modiquest) and Oscar Hoogteijling (Business Development Manager, Bioceros).
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-line-development-services-market-2017-2027/171.html
or email sales@rootsanalysis.com
Roots Analysis is a specialist market research company, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you'd like us to help you with your growing business needs, get in touch at info@rootsanalysis.com
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-line-development-services-market-2017-2027/171.html
or email sales@rootsanalysis.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release CELL LINE DEVELOPMENT SERVICES MARKET TO WITNESS STEADY GROWTH IN THE COMING DECADE here
News-ID: 984345 • Views: …
More Releases from Roots Analysis
The Agricultural Biologicals Market Size Worth over USD 40 Billion in 2035 | Roo …
Global Agricultural Biologicals Market Overview
The agricultural biologicals market is a rapidly growing and dynamic industry that is poised to grow at a CAGR of 9.98% in the forecast period 2023-2035. The growth in the agricultural biologicals market size over the next decade is likely to be the result of a rise in the demand for organic food coupled with the growing focus on sustainable agriculture. Agricultural biologics include a vast…
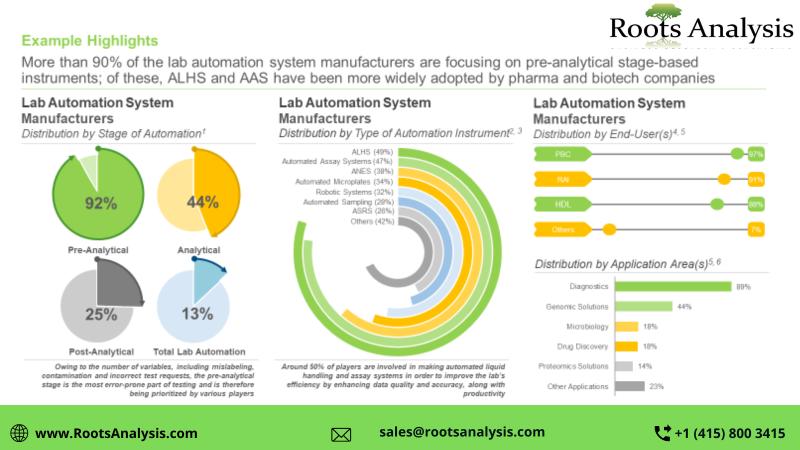
The Lab Automation Market is anticipated to grow at a CAGR of around 15% by 2035 …
Owing to the advanced features and cost-saving potential of automation, stakeholders in the healthcare industry are adopting lab automation systems in order to get precise results and reduce deviations occurring due to manual handling
Roots Analysis has announced the addition of "Lab Automation Market, 2023-2035" report to its list of offerings.
The diverse applications of lab automation systems, including advanced data management, high reproducibility and increased productivity in the biotechnology…
The gene therapy market is projected to be worth USD 17.3 billion in 2035, growi …
Given the potential of gene therapies to permanently cure a diverse array of clinical conditions at the genetic level, the current pipeline is rapidly growing; a number of such therapies are currently being evaluated in late stages of development
London
Roots Analysis has announced the addition of "Gene Therapy Market" report to its list of offerings.
Post the emergence of blockbuster gene therapies, such as ZOLGENSMA® (for spinal muscular atrophy type…

The companion diagnostics development services market, is anticipated to grow at …
Roots Analysis has announced the addition of "Companion Diagnostics Development Services Market (2nd Edition), 2022-2035" report to its list of offerings.
The growing pipeline of patient-centric targeted therapies has led to a surge in demand for companion diagnostics; these tests are known to improve the success rates of late-stage trials by almost three-fold. The development and approval of these FDA classified high-risk devices requires multidisciplinary expertise and an established network of…