Press release
Fertility and Pregnancy Rapid Test Kits Market is Expected to Generate Huge Profits by 2025
Human chorionic gonadotropin (hCG) hormone, luteinizing hormone (LH), and follicle stimulating hormone (FSH) are some of the reproductive hormones which are quantitatively detected using the tools of fertility along with pregnancy rapid test kits. In males, the detection of the sperm count can be done with the use of fertility rapid test kits. In females, the fertility rapid test kits are used to confirm menopause or to find the exact day of ovulation. Immunoassay techniques which change the color are used in these devices, the type of the device being used decides which sample should be used for the test, whether blood or urine sample, a small quantity of sample is used for the test.Increasing incidence of gynecological disorders and infertility and fast and self-contained tests are some of the driving factors for the growth of the global fertility and pregnancy rapid test kits market. Rise in first-time pregnancy age and availability of less time-consuming decentralized diagnostic tests in place of laboratory tests have boosted the market growth. Increasing inclination toward the smartphone app connectivity, attractive product features, and technological advancements have led to the adoption of fertility and pregnancy rapid test kits. These factors are boosting the demand for these devices and the market is projected to grow significantly during the forecast period. However, the impeding growth factors in countries who have inherent potential due to which these products are not well adopted, too many options in the market being available and controversies and issues related to the devices lead to the restraints of the growing market for fertility as well as pregnancy rapid test kits.
Report Overview and TOC @ https://www.transparencymarketresearch.com/fertility-pregnancy-rapid-test-kits-market.html
In Asia Pacific and Europe, the premier companies consolidate their position with strong local companies by maintaining distribution agreements and with e-commerce partners the strategies like creating alliances are employed in the market for fertility including pregnancy rapid test kits.
On the basis of product type, the market can be segmented as fertility rapid test kits and pregnancy rapid test kits. The pregnancy rapid test kits segment can be further classified as digital devices and line-indicator devices. The line-indicator devices includes mid-stream devices, strip/dip sticks, and cassettes. The fertility rapid test kits segment can be categorized as digital devices and line-indicators. On the basis of test type, the market can be segmented as hCG urine test, hCG blood test, FSH urine test, and LH urine test.
Request brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=26795
Globally, there is a high prevalence of infertility rate and gynecological disorders leading to high demand for hCG rapid test. Based on distribution channel, the market can be segmented as fertility and gynecology clinics, hypermarkets and supermarkets, online sales, drugstores, pharmacies, and other clinics. The drugstores segment is the most preferable distribution channel, followed by the pharmacies segment. However, in some regions such as North America and Europe, these traditional distribution channels have started facing significant competition from the online sales segment due to increasing consumer preference toward online sales that provide doorstep services.
Geographically, the market for fertility and pregnancy rapid test kits is distributed over North America, Europe, Asia Pacific, and rest of the world. North America dominates the global market with the U.S. accounting for a considerable market share. Europe followed North America in terms of market share. The Asia Pacific and rest of the world markets are projected to expand at significant growth rate during the forecast period attributed to factors such as high unmet needs, emerging economies, and continuously improving scenario.
for Specific and Customized requirements@ https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=26795
Prominent players operating in the fertility and pregnancy rapid test kits market include Quidel Corporation; Alere Inc.; Church & Dwight Co., Inc.; Prestige Brands Holdings, Inc.; Geratherm Medical AG; bioMérieux SA; Procter & Gamble Co.; Abbott Laboratories; Swiss Precision Diagnostics GmbH; and DCC Plc.
The report offers a comprehensive evaluation of the market. It does so via in-depth qualitative insights, historical data, and verifiable projections about market size. The projections featured in the report have been derived using proven research methodologies and assumptions. By doing so, the research report serves as a repository of analysis and information for every facet of the market, including but not limited to: Regional markets, technology, types, and applications.
Pre Book full report@ https://www.transparencymarketresearch.com/checkout.php?rep_id=26852<ype=S
About TMR
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information.
Contact
Transparency Market Research
State Tower
90 State Street,
Suite 700,
Albany NY - 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
TMR Blog: http://www.theglobalhealthnews.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Fertility and Pregnancy Rapid Test Kits Market is Expected to Generate Huge Profits by 2025 here
News-ID: 847906 • Views: …
More Releases from Transparency Market Research

Handheld Marijuana Vaporizer Market Key Drivers, Market Research, and Insights f …
The global handheld marijuana vaporizer market has witnessed remarkable growth in recent years, attributed to the increasing acceptance of medicinal marijuana and the demand for convenient and discreet consumption methods. Valued at US$ 5 billion in 2021, the market is projected to surge at a CAGR of 13.4% to reach US$ 15.9 billion by 2031. This article delves into the factors driving this growth, the evolving market landscape, and the…
Disinfectant Wipes Market Forecast 2020-2030 - Market Size, Drivers, Trends, And …
The COVID-19 pandemic has precipitated a remarkable surge in the demand for disinfectant products, with a notable emphasis on disinfectant wipes as an essential tool in maintaining hygiene and preventing the spread of pathogens. This research report delves into the multifaceted landscape of the disinfectant wipes market, elucidating key trends, drivers, and innovations shaping the industry.
𝐆𝐞𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐭𝐡𝐢𝐬 𝐫𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐫𝐞𝐩𝐨𝐫𝐭 @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=70359
Key Players and Market Developments
Key players…
Tissue Banking Market is Expected to Reach US$ 2,903.3 Million to 2026| TMR Stud …
The global 𝐭𝐢𝐬𝐬𝐮𝐞 𝐛𝐚𝐧𝐤𝐢𝐧𝐠 𝐦𝐚𝐫𝐤𝐞𝐭 reached a value of US$ 1,056.4 million in 2017 and is projected to nearly triple to US$ 2,903.3 million by 2026, with a robust Compound Annual Growth Rate (CAGR) of approximately 12.0% from 2018 to 2026. Factors such as increasing awareness about tissue donation, technological advancements, and a growing target patient population are anticipated to propel market growth during this period.
Moreover, the market is expected…
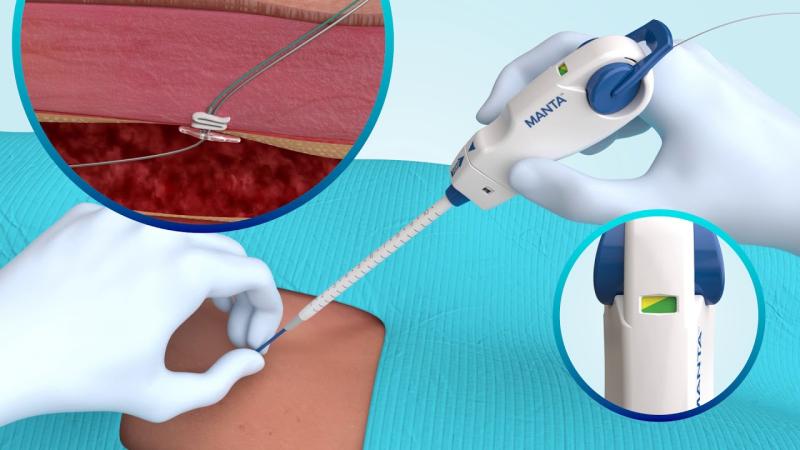
Vascular Closure Devices Market to reach US$ 1 Billion by 2027: TMR Study
This report by Transparency Market Research, Inc. assesses the present state and future growth potential of the global 𝐯𝐚𝐬𝐜𝐮𝐥𝐚𝐫 𝐜𝐥𝐨𝐬𝐮𝐫𝐞 𝐝𝐞𝐯𝐢𝐜𝐞𝐬 𝐦𝐚𝐫𝐤𝐞𝐭. It features a comprehensive executive summary, offering insights into various segments of the market. Additionally, the report provides detailed analysis and data on product, access type, application, end user, and regional segments within the global market.
Vascular closure devices is estimated to reach a value of ~US$ 1 Bn…
More Releases for Pre
Pre-Shipment Inspection India, Pre-Shipment Inspection, PSI Certification, CE Ce …
Ravi Energie is a mandated PSI Agency and a Pre-shipment Inspection Certificate from Ravi Energie is required for Customs Clearance in the “Member Countries”. Once a pre shipment inspection entity and an exporter agree on an inspection date, the pre shipment inspection entity conducts the inspection on that date unless it is rescheduled on a mutually agreed basis between the exporter and the pre shipment inspection entity. Pre-shipment Inspection (PSI)…
PSI Certification, Pre Shipment Documents, CE Certification, Industrial Pre Ship …
Ravi Energie Inc has been providing multi-level and multi-disciplinary services related to International Trade and Energy since 1976. Broadly Categorizing, Ravi Energie has a unique global network and being an inspection agency that's just around the corner, we provide services at virtually every harbor and airport around the world. Ravi Energie understands each country's policy and accordingly checks for the implementation and gives a certification.
Ravi Energie provide a real competitive…
Pre-Shipment Inspection, Pre Shipment Certification, Certification, Pre Shipment …
Ravi energie Inc offers fast, expert product testing, inspection and certification solutions through our global network of accredited laboratories. Ravi energie Inc. supports manufacturers of energy generation and distribution equipment with testing and certification solutions for access to global markets. Whether you're engaged in fossil fuel or renewable energy development,
Ravi Energie which is an ISO 9001 certified company Customs classification is verified for each imported item to allow Customs…
Pre-Shipment Inspection Certification, Pre Shipment Verification, Pre Shipment C …
Ravi Energie also issues Clean Report of Findings (CRF) after inspection which specifying that the goods have been inspected before shipment and the price of the goods exported has been verified. In most PSI-using countries, physical inspection and price verification of almost all goods prior to exportation is obligatory for imports to be permitted today over 30 countries in Africa, Asia and Latin America use Pre-shipment Inspection Certification services.
In India…
Pre-Shipment Inspection Certification, Pre Shipment Certification, Pre Shipment …
Ravi Energie Provide Pre-Shipment Inspection Certification, Pre Shipment Documents as being mandated PSI Agency and a PSI Certificate from us is required for Customs Clearance in the Member Countries. Pre shipment inspection activities are all activities relating to the verification of the quality, quantity. In most PSI-using countries, physical inspection and price verification of almost all goods prior to exportation is obligatory for imports to be permitted today over 30…
CE Certification, Industrial Pre Shipment Inspection, PSI Certification, Pre Shi …
Ravi Energie is an ISO 9001 c`ertified USA & India based company and authorized to provide services like Pre-Shipment Inspection. Pre-shipment inspection includes the checking of the commodity against any list of items subject to import regulations. Customs classification is verified for each imported item to allow Customs to apply the correct tariff rates.
The fact of an import-driven economy made it essential to monitor the quantity and quality of goods…
