Press release
Dural Arteriovenous Fistulas Treatment Global Market 2017: Key Players – BD, Medtronic, B. Braun Melsungen AG, Cook, Teleflex Incorporated and More
Executive SummaryMarket Research Future published new report, titled “Dural Arteriovenous Fistulas Treatment Market -Research Report: Global Forecast till 2023”.
Dural Arteriovenous Fistulas (DAVF) Treatment Market Information: By Type (Type I, Type II, Type III), Treatment (Surgery, Stereotactic Radiosurgery, Embolization), End User (Hospitals & Clinics, Ambulatory Surgical Centers) - Global Forecast till 2023
The global dural arteriovenous fistulae treatment market is expected to grow at a CAGR of ~ 7.68 % during the forecast period 2017-2023.
GET SAMPLE REPORT @ https://www.marketresearchfuture.com/sample_request/4580 .
Market Synopsis of Global Dural Arteriovenous Fistulas Treatment Market:
Dural arteriovenous fistulae (DAVF) is caused by abnormal connections between arteries and veins in a protective membrane on the outer layer of the brain and spine; it is a rare disease. These abnormal blood vessels divert blood from the normal paths. If the volume of diverted blood flow is large, tissue downstream may not receive an adequate blood and oxygen supply. The symptoms vary, depending on the location of fistulae. Headaches are among the most common symptoms with all types of DAVFs. Other symptoms include visual problems, ringing in ears, heart strokes, and others.
Increasing prevalence of brain injury and neurological disorders, increasing investment in biotechnology and pharmaceutical industries in R&D, and rising need for the better treatment methods drive the growth of the market. Moreover, favorable reimbursement policies, increased applications, and significant investments in the development of advance technologies for the treatment methods likely to fuel the market growth during the forecast period 2017-2023.
As per the Centers for Disease Control and Prevention in 2014, about 2.8 million TBI-related Emergency Department (ED) visits, hospitalizations, and deaths occurred in the United States. TBI (traumatic brain injury) contributed to the deaths of nearly 50,000 people. It was diagnosed in over 282,000 hospitalized cases and over 2.5 million ED visits. These consisted of TBI alone or TBI in combination with other injuries. Adults aged over 75 years have the highest rates of TBI-related hospitalization and deaths.
According to the WHO estimates, neurological disorders are responsible for 4.5%-11% of all illnesses including low or high income economies. This is far higher as compared to the number of gastrointestinal disorders, or cancers, and is expected to increase further over the coming years.
However, higher cost of these treatment procedure may hamper the market growth during the corresponding period.
Some of Key Players:
Some of key the players in the market are BD (U.S.), Medtronic (U.S.), B. Braun Melsungen AG (Germany), Cook (U.S.), Teleflex Incorporated (U.S.), Fresenius Medical Care AG & Co. KGaA (Germany), NxStage Medical, Inc.(U.S.), and Poly Medicure Limited.
Segmentations:
The global dural arteriovenous fistulae treatment market is segmented on the basis of type, treatment, and end user.
On the basis of type, the market is segmented into type I, type II, and type III.
On the basis of application, the market is segmented into surgery, stereotactic radiosurgery, embolization, and others.
On the basis of end user, the market is segmented into hospitals & clinics, ambulatory surgical centers, and others.
GET FULL REPORT @ https://www.marketresearchfuture.com/reports/dural-arteriovenous-fistulas-treatment-market-4589 .
Regional Analysis:
The Americas dominates the global dural arteriovenous fistulae treatment market owing to developing technology, increasing cases of brain injury, rising prevalence of neurological disorders, growing healthcare spending, and increasing government support for research & development. Furthermore, increasing R&D activities and the presence of major companies have fuelled the growth of the market in this region.
Europe holds the second position in the global dural arteriovenous fistulae treatment market. It is expected that the government support for research & development and availability of funds for research may drive the market in European region over the review period.
Asia Pacific is the fastest growing dural arteriovenous fistulae treatment market owing to the presence of rapidly developing healthcare technology, huge patient population, and high healthcare expenditure. Moreover, increasing new opportunities in countries such as China, India, Japan, and South Korea projected to emerge as the fastest growing market across the globe. Furthermore, increasing demand for the neurological treatment may lead to the use of advanced equipment, which, in turn, will increase the growth of the dural arteriovenous fistulae treatment in the region.
On the other hand, the Middle East and Africa holds the least share of the market due to limited medical facilities, lack of funds for research and development, and poor political conditions, especially, in Africa.
GET DISCOUNT @ https://www.marketresearchfuture.com/check-discount/4580 .
Table of Contents:
Chapter 1. Report Prologue
Chapter 2. Market Introduction
2.1 Definition
2.2 Scope Of The Study
2.2.1 Research Objective
2.2.2 Assumptions
2.2.3 Limitations
Chapter 3. Research Methodology
3.1 Introduction
3.2 Primary Research
3.3 Secondary Research
3.4 Market Size Estimation
Chapter 4. Market Dynamics
4.1 Drivers
4.2 Restrains
4.3 Opportunities
4.4 Challenges
4.5 Macroeconomic Indicators
4.6 Technology Trends & Assessment
...
Chapter 11. Company Profiles
11.1 BD
11.1.1 Company Overview
11.1.2 Product Overview
11.1.3 Financials
11.1.4 SWOT Analysis
11.2 Medtronic
11.2.1 Company Overview
11.2.2 Product Overview
11.2.3 Financial Overview
11.2.4 Key Developments
11.2.5 SWOT Analysis
11.3 B. Braun Melsungen AG
11.3.1 Company Overview
11.3.2 Product Overview
11.3.3 Financial Overview
11.3.4 Key Development
11.3.5 SWOT Analysis
11.4 Cook
11.4.1 Company Overview
11.4.2 Product/Business Segment Overview
11.4.3 Financial Overview
11.4.4 Key Development
11.4.5 SWOT Analysis
11.5 Teleflex Incorporated
11.5.1 Company Overview
11.5.2 Product Overview
11.5.3 Financial Overview
11.5.4 Key Developments
11.5.5 SWOT Analysis
11.6 Fresenius Medical Care AG & Co. KGaA
11.6.1 Company Overview
11.6.2 Product Overview
11.6.3 Financial Overview
11.6.4 Key Developments
11.6.5 SWOT Analysis
11.7 NxStage Medical, Inc.
11.7.1 Overview
11.7.2 Product Overview
11.7.3 Financials
11.7.4 Key Developments
11.7.5 SWOT Analysis
11.8 Others
Chapter 12. MRFR Conclusion
12.1 Key Findings
12.1.1 From CEO’s View Point
12.1.2 Unmet Needs Of The Market
12.2 Key Companies To Watch
12.3 Prediction Of Medical Devices Industry
...CONTINUED
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions.
In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.
Contact:
Akash Anand,
Market Research Future
Office No. 528, Amanora Chambers
Magarpatta Road, Hadapsar,
Pune - 411028
Maharashtra, India
+1 646 845 9312
Email: salesteam@marketresearchfuture.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Dural Arteriovenous Fistulas Treatment Global Market 2017: Key Players – BD, Medtronic, B. Braun Melsungen AG, Cook, Teleflex Incorporated and More here
News-ID: 795433 • Views: …
More Releases from Market Research Future

Hydrofluoric Acid Market (CAGR) of 4%, Innovation Imperative Future Proofing You …
Hydrofluoric acid (HF) is a crucial chemical compound with a wide range of applications across various industries. Despite its hazardous nature, it plays an essential role in manufacturing processes, especially in the production of fluorine compounds. The global hydrofluoric acid market has been witnessing steady growth, driven by demand from end-user industries such as oil refining, pharmaceuticals, and electronics.
The Hydrodesulfurization Catalysts Market is projected to register a CAGR of over…
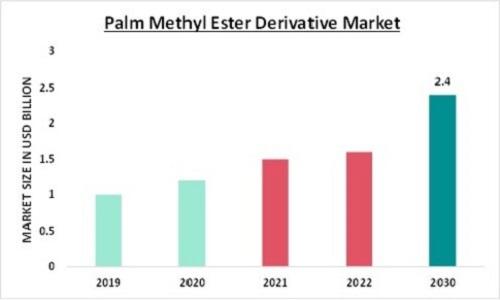
Palm Methyl Ester Derivative Market Size Projected to Grow at 5.92% CAGR, Reachi …
In recent years, the global market for palm methyl ester derivatives has witnessed significant growth, driven by various factors including environmental concerns, technological advancements, and the increasing demand for sustainable alternatives in various industries. Palm methyl ester derivatives, derived from palm oil, have emerged as versatile ingredients with applications spanning across sectors such as cosmetics, pharmaceuticals, lubricants, and more.
The Palm Methyl Ester Derivative Market Size was valued at USD 1.5…

Asia-Pacific Ceramic Tiles Market to Register Highest CAGR Growth of 7.50% by 20 …
The Asia-Pacific ceramic tiles market has been witnessing robust growth, driven by various factors such as increasing investments in residential and commercial construction, renovation activities, and the growing preference for aesthetically pleasing and durable flooring solutions. Countries like China, India, Japan, and South Korea have been leading the market growth, supported by strong manufacturing capabilities, technological advancements, and evolving consumer preferences.
Asia-Pacific Ceramic Tiles Market Size was valued at USD 141.2…

APAC Wallpaper Market to Register Highest CAGR Growth of 8% by 2032: Analysis by …
The APAC wallpaper market has witnessed significant growth in recent years, driven by factors such as rising disposable incomes, expanding construction activities, and growing awareness regarding interior decor. Countries like China, India, Japan, South Korea, and Australia have emerged as key contributors to the region's wallpaper market. Additionally, the increasing adoption of innovative wallpaper designs and patterns is fueling market growth further.
APAC Wallpaper Market Size was valued at USD 0.55…
More Releases for Overview
Global Front Loading Wheel Dumper Market Overview, Product Overview, Segmentatio …
Global Front Loading Wheel Dumper Market Overview:
The global Front Loading Wheel Dumper market is expected to grow at a significant pace, reports QY Research. Its latest research report, titled [name of the report], offers a unique point of view about the global market. Analysts believe that the changing consumption patterns are expected to have a great influence on the overall market. For a brief overview of the global Front…
Perfumes Market Overview, Product Overview and Market Segmentation (Edition 2020 …
Global Perfumes Market, delivering a must-read report for industry stakeholders wanting to understand the strategic landscape of this increasing sector. Readers will find an in-depth analysis of the market and how it will impact existing traditional markets, as well as insights into future development and opportunities across the globe.
The global perfumes market was worth $30.6 billion in 2019. It is expected to grow at a compound annual growth rate (CAGR) of…
Telehandlers Market 2017-2027 | Drivers and Restraints, Segmentation, Overview, …
The telehandlers also known as telescopic handlers are revolutionary machineries in the 20th century that are proven to be beneficial in varied range of industries across the world. With the innovations taking place coupled with the growing industrialization, the telehandlers market find wide applications in construction, agricultures and especially in transfer station, rib handler and etc. The telehandlers market offers various products based on the end users requirement where telehandlers are…
Digital X-Ray Machine Market Overview, Product Overview, Segmentation, Overview …
The Digital X-Ray Machine market revenue was xx.xx Million USD in 2013, grew to xx.xx Million USD in 2017, and will reach xx.xx Million USD in 2023, with a CAGR of x.x% during 2018-2023. Based on the Digital X-Ray Machine industrial chain, this report mainly elaborate the definition, types, applications and major players of Digital X-Ray Machine market in details. Deep analysis about market status (2013-2018), enterprise competition pattern, advantages…
Global Chromite Market Overview, Industry Analysis, Demand, Overview & Forecast …
An up-to-date research report 2018 has been disclosed by Market Research Hub highlighting the title “Global Chromite Market Report 2018” which provides an outlook for current market value as well as the expected forecast including Rate on Investment (ROI) together with the growing CAGR near XX% during 2018-2025. The report studies the Chromite market worldwide, especially in North America, China, Europe, Southeast Asia, Japan and India, with production, size, growth,…
Strutfast Company Overview
Established in Johannesburg, South Africa in 2001, STRUTFAST designs, manufactures and supplies cost effective cable management systems for all industries. The focus of the company is to provide high quality solutions to meet and exceed even the most arduous project requirements, especially for mining and power applications. With an emphasis on innovation & full project lifecycle support, Strutfast has the technical design skills, manufacturing capability and logistics expertise to ensure…
