Press release
Recent Research: Digital Companion Diagnostics Market Analysis to 2025
Companion diagnostics (CD) refer to a type of in vitro diagnostic tests or assays that are intended to help physicians in making effective treatment decisions based on the patient’s response to a specific drug targeted toward a specific biomarker, expressed in a particular disease or indication. The patient’s response to an ongoing therapy is mapped with the help of companion diagnostics in terms of changes in gene amplification, nullifying the effect of mutations, changes in protein overexpression and receptor-substrate interaction, and so on.These tests aim at providing insights into the patient’s likely response pattern to a particular therapy, thereby enabling optimization of appropriate dosing concentrations and course of administration. The increasing importance of personalized medicine and commercialization of targeted therapeutics have driven the demand for companion diagnostics, which in turn is projected to observe a rise in trend of adoption by patients as well as health care service providers.
Obtain Report Details @ http://www.transparencymarketresearch.com/digital-companion-diagnostics-market.html
Companion diagnostics help in cost reduction for drug manufacturers by narrowing the target patient population who are likely to benefit from the targeted therapy. These tests enable physicians to rule out therapeutic options which are not likely to be as effective as a particular targeted drug, and thus eliminate the need for use of higher-priced cancer therapeutics and other unnecessary treatments. The consideration of a defined patient population and resultant higher efficacy of targeted therapeutics further increase the chances of the drug’s approval and aid in streamlining of the drug development process (such as fasten the approval process). In addition, companion diagnostics are important to rule out the possibility of side effects and alleviate health care costs related to management of side effects.
The demand for personalized medicine has been on a rise since the significant development in genomics research and biomarkers. This can be attributed to the efficiency and target-specific nature of personalized medicine with better patient outcomes. In addition, the targeted therapies have minimal or no side effects compared to the conventional therapies. With the paradigm shift in the drug discovery and development process, pharmaceutical companies are shifting their focus toward the development of novel targeted therapies (genotype or phenotype specific therapies).
The adoption of this paradigm shift marked the gradual termination of traditional blockbuster model. Hence, these factors increase the demand and uptake of personalized medicine which in turn drives the growth of the companion diagnostics market. Increase in the number of cancer incidence, demand for personalized medicine, and reduction in drug development cost have contributed to augment the growth of the digital companion diagnostics market. However, poor reimbursement policies, and longer developmental and approval phase are factors that are restraining the growth of the market.
The market for digital companion diagnostics can be segmented on the basis of technology, disease indication, end-user, and geography. Based on technology, it is segmented into molecular diagnostics and immunohistochemistry. The molecular diagnostics segment is further sub-segmented into PCR, in situ hybridization, and next generation sequencing. This segment is anticipated to be a fast growing market due to advancement in technology, adoption of precise and accurate techniques by users, and reduction of cost and time associated with use of these methods.
Based on disease indication, the market is segmented into breast cancer, melanoma, lung cancer, colorectal cancer, and infectious disease. The breast cancer segment is estimated to hold a large market size during the forecast period. The major factors fueling growth of this market are rise in global incidence rates of breast cancer, shift toward development of targeted therapies, and increase in acceptance level of the test due to the enhanced specificity and efficiency.
Make an Enquiry @ http://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=20603
Based on end-user, the market is segmented into hospitals, pharmaceutical companies, and laboratories. Based on geography, the market for digital companion diagnostics has been distributed over North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is estimated to dominate the market due to increase in health care expenditure and rise in demand for personalized treatment.
Key potential players operating in the digital companion diagnostics market are Qiagen N.V., Agilent Technologies, Inc., Agendia N.V., Life Technologies Corporation, GE Healthcare Ltd., Roche Holdings AG, Abbott Laboratories, and Genomic Health, Inc.
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Contact Us
Transparency Market Research
State Tower,
90 State Street, Suite 700
Albany, NY 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Recent Research: Digital Companion Diagnostics Market Analysis to 2025 here
News-ID: 648880 • Views: …
More Releases from Transparency Market Research

Handheld Marijuana Vaporizer Market Key Drivers, Market Research, and Insights f …
The global handheld marijuana vaporizer market has witnessed remarkable growth in recent years, attributed to the increasing acceptance of medicinal marijuana and the demand for convenient and discreet consumption methods. Valued at US$ 5 billion in 2021, the market is projected to surge at a CAGR of 13.4% to reach US$ 15.9 billion by 2031. This article delves into the factors driving this growth, the evolving market landscape, and the…
Disinfectant Wipes Market Forecast 2020-2030 - Market Size, Drivers, Trends, And …
The COVID-19 pandemic has precipitated a remarkable surge in the demand for disinfectant products, with a notable emphasis on disinfectant wipes as an essential tool in maintaining hygiene and preventing the spread of pathogens. This research report delves into the multifaceted landscape of the disinfectant wipes market, elucidating key trends, drivers, and innovations shaping the industry.
𝐆𝐞𝐭 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐭𝐡𝐢𝐬 𝐫𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐫𝐞𝐩𝐨𝐫𝐭 @ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=70359
Key Players and Market Developments
Key players…
Tissue Banking Market is Expected to Reach US$ 2,903.3 Million to 2026| TMR Stud …
The global 𝐭𝐢𝐬𝐬𝐮𝐞 𝐛𝐚𝐧𝐤𝐢𝐧𝐠 𝐦𝐚𝐫𝐤𝐞𝐭 reached a value of US$ 1,056.4 million in 2017 and is projected to nearly triple to US$ 2,903.3 million by 2026, with a robust Compound Annual Growth Rate (CAGR) of approximately 12.0% from 2018 to 2026. Factors such as increasing awareness about tissue donation, technological advancements, and a growing target patient population are anticipated to propel market growth during this period.
Moreover, the market is expected…
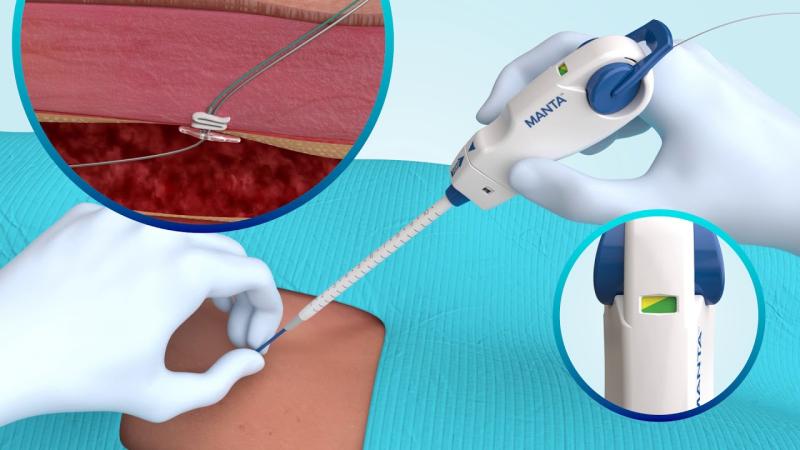
Vascular Closure Devices Market to reach US$ 1 Billion by 2027: TMR Study
This report by Transparency Market Research, Inc. assesses the present state and future growth potential of the global 𝐯𝐚𝐬𝐜𝐮𝐥𝐚𝐫 𝐜𝐥𝐨𝐬𝐮𝐫𝐞 𝐝𝐞𝐯𝐢𝐜𝐞𝐬 𝐦𝐚𝐫𝐤𝐞𝐭. It features a comprehensive executive summary, offering insights into various segments of the market. Additionally, the report provides detailed analysis and data on product, access type, application, end user, and regional segments within the global market.
Vascular closure devices is estimated to reach a value of ~US$ 1 Bn…
More Releases for Companion
Companion Animal Drugs - Key Market Statistics
Companion animals are pets kept primarily for company, entertainment, and safety. Owners of companion animals consider them to be family, friends, and confidants. Dogs, cats, birds, rabbits, horses, goats, gerbils, rats, mice, and amphibians, others are considered as companion animals.
Read Report Overview - https://www.transparencymarketresearch.com/companion-animal-specialty-drugs-market.html
According to the American Society for the Prevention of Cruelty to Animals (ASPCA) defines companion animal to be domesticated or domestically-bred whose emotional, physical, social, and behavioral…
Companion Animal Vaccine – Improving The Health Of Companion Animals In A Cost …
The companion animal vaccines market is expected to witness significant growth during the forecast period due to launch of vaccines for companion animals. For instance, in December 2017, Zoetis Inc. announced the launch of Vanguard CIV H3N2/H3N8, the latest vaccine in the company’s canine influenza virus (CIV) portfolio. The new bivalent vaccine helps protect dogs against the two strains of the virus known to be circulating in the U.S.
Moreover, the…
Companion Diagnostics Market - Easing of Regulatory Policies to Drive Global Dem …
Companion diagnostics include two types of tests namely tests which are developed along with target drugs and the other developed after the commercialization of drugs. Companion diagnostics combined with targeted therapeutics are being seen as a major breakthrough in the personalized medicine field. Companies operating in the companion diagnostics market are seeking an effective growth strategy in the development of this concept drug-diagnostic interaction as the healthcare sector is shifting…
Global Companion Diagnostics Market
The Global Companion Diagnostics (CDx) Market was valued at US$ 1,614.5 million in 2015 and is projected to expand at a CAGR of 12.0% during the forecast period (2016–2024), as highlighted in a new report published by Coherent Market Insights. Development of multiple biomarkers and targeted drug therapy is boosting research and development collaboration among the industry players. This is expected to improve the time to market for companion diagnostic (CDx) test…
Global Companion Diagnostics – Growth Companion for Personalized Medicine
Companion Diagnostics: Growth Companion for Personalized Medicine
Personalized medicine is the new segment in discussion among healthcare experts around the globe. Also, encouragement from the regulatory bodies such as the FDA and EMEA in erms of providing a defined structure for companion diagnostic development is driving research activities in personalized medicine. Diagnosis at molecular level for each individual is of prime importance to identify the target biomarker before deciding the therapy.…
Companion diagnostics Market: Companion diagnostic are used to determine patient …
The few companion diagnostics are already available in the market for specific types of cancer and many are already under clinical development. Companion diagnostics test depends on finding a specific biomarker in the patient and hence biomarker presence or absence determines whether or not the patient will respond to the treatment. This test require a biopsied tissue to access the expression of biomarker protein or mutant or aberrant genes. Companion…
