Press release
Artificial Pancreas Device System (APDS) - Forecast till 2024
Artificial Pancreas Device System (APDS) – Putting the Ease in Diabetes ManagementAdministering insulin on daily basis is cumbersome for type 1 diabetes (T1D) patients in a busy lifestyle. While devices such as insulin pumps, pens, and jet injectors have made living with diabetes less stressful by ridding users of relatively painful insulin delivering devices such as needles and syringes. Juvenile Diabetes Research Foundation (JDRF) is working in collaboration with many industry players such as Medtronic, Inc., Johnson & Johnson, and Tandem Diabetes Care, Inc. to develop innovative insulin and glucose monitoring systems/devices.
Artificial pancreas is the outcome of these collaborations which is also encouraged for use by the FDA. Artificial pancreas device system (APDS) consists of an insulin pump, sensor, transmitter, and a receiver. MiniMed 530G from Medtronic, Inc. was the first artificial pancreas device system (APDS) approved by FDA. This threshold suspend device system enables temporary suspension of insulin delivery when the glucose level is lower than the threshold level. Another system, Medtrum’s P6 Easy Touch Disposable Pump is a semi-closed loop artificial pancreas that is under clinical trials in Europe. Many such systems are under clinical trials and expected to be launched in near future in the global artificial pancreas device system (APDS) market. Developed economies in North America, Europe and Pacific region would be the potential regional opportunities for this novel system in the global artificial pancreas device system (APDS) market.
Request a sample copy of this report @ https://www.coherentmarketinsights.com/insight/request-sample/79
Technological advancements to drive growth of the global artificial pancreas device system (APDS) market
In 2016, the U.S. FDA approved a hybrid closed-loop artificial pancreas device system (APDS) MiniMed 670G which is an automated system. The continuous glucose monitor in the system automatically sends signals to the insulin pump to deliver insulin when the glucose level in body goes beyond the acceptable limit. Thus, there is no need for the user to stimulate the release of insulin every time the glucose level is abnormal. This provide the user freedom to perform his/her daily activities. Such advancement in T1D management is expected to be highly preferred by the users and medical community, leading to higher advocacy to use this device especially among the patient group using insulin pumps. Moreover, in mid-2018, a study will test a bihormonal “bionic pancreas” system at the Massachusetts General Hospital (Boston) and Boston University. This would further fuel the artificial pancreas device system (APDS) market growth and attract more investments.
Strategic collaborations fostering artificial pancreas device system (APDS) market growth
There were over 20 projects undertaken by the industry payers in 2015 with respect to development of artificial pancreas device system (APDS). Bigfoot Biomedical acquired Asante’s FDA approved Snap insulin pump technology in 2015 and at the same time signed a development agreement with Dexcom to integrate Dexcom’s CGM in its under development artificial pancreas device system (APDS). Dexcom is in collaboration with many other market players such as Insulet Corporation, Animas Corporation, and International Diabetes Closed Loop wherein, these companies would be incorporating Dexcom’s CGM in their artificial pancreas device system (APDS). Such collaborations are expected to expedite market entry for new artificial pancreas device system (APDS)s and thus drive the growth of artificial pancreas device system (APDS) market.
Enquire Here: https://www.coherentmarketinsights.com/market-insight/artificial-pancreas-market-79
Lack of reimbursement to hinder artificial pancreas device system (APDS) market growth
The prevalence of T1D in the U.S. is 1.35 million which is expected to increase to 5 million by 2050 as per the estimates (2016) by JDRF. Each year 40,000 people are diagnosed with T1D in the U.S. according to the JDRF. With this huge patient base, potential opportunity for artificial pancreas device system (APDS) looks considerably high at global level. However, lack of reimbursement for glucose sensors is expected to inhibit the uptake of artificial pancreas device system (APDS). With an average cost of US$ 6000-7000, adoption of artificial pancreas device system (APDS) is estimated to be restricted among rich T1D patients. Furthermore, only a few T1D patient group prefer insulin pumps for treatment. According to the 2013 report of JDRF, about 350,000 insulin pumps were sold in the U.S. Thus, creating awareness should also be an objective of artificial pancreas device system manufacturers to increase its adoption.
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: sales@coherentmarketinsights.com
Visit Blog: http://healthcaremarketconsulting.blogspot.in/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Artificial Pancreas Device System (APDS) - Forecast till 2024 here
News-ID: 466884 • Views: …
More Releases from Biotechnology Industry Report By CMI

The New Research Trial Drives The Shigella Vaccines Market
Shigella is a gram-negative pathogenic enterobacteria that causes severe diarrhea and dysentery in humans. Symptoms associated with Shigella infection includes fever, stomach pain, tenesmus, watery diarrhea, vomiting, dehydration, and convulsions. Various strains of Shigella are encompassed, such as S. dysenteriae, S. flexneri, S. sonnei and S. boydii. Each species of Shigella has different serotypes classified on the basis of the structure of O-antigens repeats that are the polysaccharides moiety of the lipopolysaccharide, a virulence…
Flow Cytometry Market to Surpass US$ 7.64 Billion Threshold by 2025
Flow Cytometry – It’s not just for immunologist anymore
Flow cytometry is a widely used method for analyzing the expression of cell surface and intracellular molecules. It can also characterize and define different cell types in a heterogeneous cell population. Moreover, flow cytometry also allows simultaneous multi-parameter analysis of single cells. The growth in flow cytometry market is mainly driven by the combination of technological improvements, growing research activities in life…
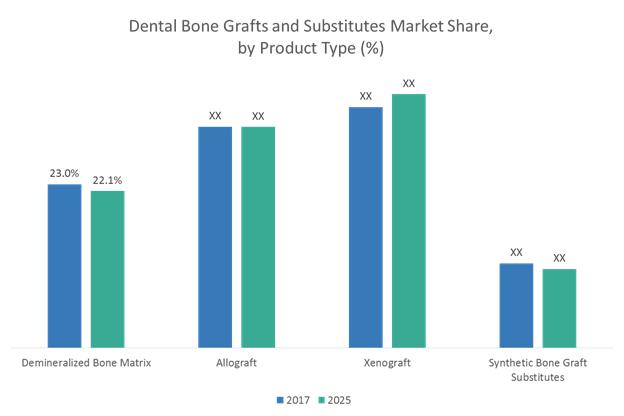
Dental Bone Graft and Substitutes Market to Surpass US$ 911.4 Million Threshold …
Dental Bone Graft and Substitutes Market Insights
Dental bone grafts are used as fillers or scaffolds that facilitate bone formation and helps in the wound healing and are suitable for a variety of clinical procedures such as filling of sockets, preservation of ridge volume (also referred to as ridge preservation), and osteogenesis i.e., the formation of new bones. Dental bone grafts are bioresorbable and are not reactive to antigen-antibody. Bone grafts…

Dental Bone Graft and Substitutes Market to Surpass US$ 911.4 Million Threshold …
Dental Bone Graft and Substitutes Market Insights
Dental bone grafts are used as fillers or scaffolds that facilitate bone formation and helps in the wound healing and are suitable for a variety of clinical procedures such as filling of sockets, preservation of ridge volume (also referred to as ridge preservation), and osteogenesis i.e., the formation of new bones. Dental bone grafts are bioresorbable and are not reactive to antigen-antibody. Bone grafts…
More Releases for APDS
Silicon Avalanche Photodiodes (Si-APDs) Market: Growth & Covid 19 Impact Analysi …
Silicon Avalanche Photodiodes (Si-APDs) Market Insights 2022 to 2030
Silicon Avalanche Photodiodes (Si-APDs) Market report provides a detailed study of important aspects such as market drivers, restraints, prospects, opportunities, market risks, current trends, and technical and industrial advancements. The report provides comprehensive analysis reports and competitive analysis reports. It provides a thorough study report that includes detailed information of Silicon Avalanche Photodiodes (Si-APDs) market size, market growth, and new players in…
Artificial Pancreas Device System (APDS) Market Growth, Trends, Industry Analysi …
The global artificial pancreas device systems market accounted for US$ 481.8 Mn in 2017 and is expected to grow at a CAGR of 20% during the forecast period 2021 - 2027, to account for US$ 2,168.1Mn by 2027.
The artificial pancreas device system consists of two types of devices for monitoring diabetes: a continuous glucose monitoring system (CGM) and an insulin infusion pump. A blood glucose meter used to calibrate the…
Global InGaAs Avalanche Photodiodes (InGaAs-APDs) Market Huge Growth Opportunity …
LP INFORMATION recently released a research report on the InGaAs Avalanche Photodiodes (InGaAs-APDs) market analysis, which studies the InGaAs Avalanche Photodiodes (InGaAs-APDs)'s industry coverage, current market competitive status, and market outlook and forecast by 2025.
Global "InGaAs Avalanche Photodiodes (InGaAs-APDs) Market 2020-2025" Research Report categorizes the global InGaAs Avalanche Photodiodes (InGaAs-APDs) market by key players, product type, applications and regions,etc. The report also covers the latest…
Global Artificial Pancreas Device System Market | APDS Industry Report Forecasts …
Global Artificial Pancreas Market Analysis By Type CTR (control to range) and CTT (control to target); By End User (hospitals, medical centers, and others); by Geography (North America, Europe, Asia Pacific, Latin America and Middle East & Africa) to 2025.
The report provides forecast and analysis of the global artificial pancreas market. It provides market overview of global artificial pancreas Market of in terms of revenue (in US$ Mn). Furthermore, the…
Artificial Pancreas Device System (APDS) Market - Global Industry Insights, Tren …
Administering insulin on daily basis is cumbersome for type 1 diabetes (T1D) patients in a busy lifestyle. While devices such as insulin pumps, pens, and jet injectors have made living with diabetes less stressful by ridding users of relatively painful insulin delivering devices such as needles and syringes. Juvenile Diabetes Research Foundation (JDRF) is working in collaboration with many industry players such as Medtronic, Inc., Johnson & Johnson, and Tandem…
Global Artificial Pancreas Device System (APDS) Market Forecast till 2024
Artificial Pancreas Device System (APDS) – Putting the Ease in Diabetes Management
Administering insulin on daily basis is cumbersome for type 1 diabetes (T1D) patients in a busy lifestyle. While devices such as insulin pumps, pens, and jet injectors have made living with diabetes less stressful by ridding users of relatively painful insulin delivering devices such as needles and syringes. Juvenile Diabetes Research Foundation (JDRF) is working in collaboration with many…