Press release
Germany Pharmacovigilance Market to grow at 12.43% CAGR | North America leads with 35% share | Top Companies - ArisGlobal, BioClinica, Cognizant
Leander, Texas and Tokyo, Japan - Feb.06.2026As per DataM intelligence research report "The Global Pharmacovigilance Market is expected to grow at a CAGR of 12.43% during the forecast period 2024-2031."
Growth is supported by increasing drug safety regulations and rising clinical trial volumes. Software-based pharmacovigilance solutions dominate adoption. Pharmaceutical companies and CROs are primary end-users. Regulatory compliance and real-world evidence monitoring drive market expansion.
Download your exclusive sample report today: (corporate email gets priority access):
https://www.datamintelligence.com/download-sample/pharmacovigilance-market?prasad
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Pharmacovigilance Market: Competitive Intelligence
ArisGlobal, BioClinica, Capgemini, Cognizant, FMD K&L Inc., Foresight Group International AG, IBM Corporation
In the Pharmacovigilance Market, a group of established and innovative firms ArisGlobal, BioClinica, Capgemini, Cognizant, FMD K&L Inc., Foresight Group International AG, and IBM Corporation are collectively strengthening safety surveillance and regulatory compliance across the life sciences value chain. These companies provide critical services and technologies that enhance drug safety reporting, signal detection, and risk management for pharmaceutical and biotech sponsors worldwide. By integrating advanced analytics, regulatory expertise, and global operations, they help reduce adverse event response times and improve patient safety outcomes. Their efforts support life sciences organizations in navigating increasingly complex pharmacovigilance requirements, contributing to safer products and more resilient health systems.
Individually and together, these firms propel the Pharmacovigilance Market's evolution toward data‐driven, scalable, and compliant safety ecosystems. Capgemini, and Cognizant bring extensive consulting and managed services capabilities that help sponsors modernize safety operations and implement end‐to‐end pharmacovigilance solutions. ArisGlobal, BioClinica, and FMD K&L Inc. specialize in core safety case management, regulatory submission support, and compliance workflows that increase efficiency and transparency. Foresight Group International AG contributes strategic insights and service coverage that enhance global safety intelligence, while IBM Corporation's technology platforms including AI and cognitive computing empower predictive safety signal detection and automation, adding competitive advantage.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/pharmacovigilance-market?prasad
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Technological Advancements
✅ Feb 2026 - AI-Driven Signal Detection in Drug Safety Monitoring
Pharmacovigilance solution providers advanced AI and machine-learning models to improve early detection of adverse drug reaction signals using real-world data and spontaneous reporting systems.
✅ Jan 2026 - Integration of Real-World Evidence into PV Platforms
Life sciences companies enhanced pharmacovigilance platforms by integrating electronic health records and real-world evidence sources to strengthen post-market safety surveillance.
✅ Nov 2025 - Automation of Case Processing and Regulatory Reporting
Pharmacovigilance teams increased adoption of automated case intake, triage, and regulatory submission workflows to improve compliance and reduce manual processing time.
Product Launches & Innovations
✅ Feb 2026 - Launch of Cloud-Based End-to-End PV Platforms
Technology vendors introduced cloud-native pharmacovigilance platforms offering end-to-end case management, signal detection, and regulatory reporting across global markets.
✅ Dec 2025 - Advanced Natural Language Processing for Safety Data
New NLP-enabled tools were launched to extract adverse event information from unstructured sources such as clinical narratives, call center logs, and medical literature.
✅ Nov 2025 - Risk Management and Benefit-Risk Assessment Tools
Pharmacovigilance software providers released enhanced risk management modules to support benefit-risk evaluation and periodic safety update reporting.
Mergers & Acquisitions
✅ Jan 2026 - Strategic Acquisition of PV Technology Specialist
A life sciences technology company acquired a pharmacovigilance software specialist to expand its drug safety and regulatory compliance capabilities.
✅ Dec 2025 - Consolidation of PV Services Providers
Two pharmacovigilance service organizations completed a merger to broaden global coverage and strengthen outsourced drug safety operations.
✅ Nov 2025 - CRO Expands Pharmacovigilance Capabilities Through Acquisition
A contract research organization acquired a regional pharmacovigilance firm to enhance post-market surveillance and regulatory support services.
Segment Covered in the Pharmacovigilance Market:
By Service Provider
The market is segmented into Contract Outsourcing 65% and In-house 35%, with Contract Outsourcing dominating due to increasing complexity of drug safety regulations, cost efficiency, and growing reliance on specialized CROs and pharmacovigilance service providers. Pharmaceutical companies increasingly outsource adverse event reporting, risk management, and regulatory compliance activities. In-house pharmacovigilance remains relevant for large pharma companies with established internal safety teams. Rising clinical trial volumes support demand across both models.
By Type of Reporting
Based on type of reporting, the market includes Spontaneous Reporting 35%, Intensified ADR Reporting 25%, Cohort Event Monitoring 15%, Targeted Spontaneous Reporting 15%, and EHR Mining 10%, with Spontaneous Reporting leading due to its widespread adoption by regulatory authorities and healthcare professionals for post-marketing surveillance. Intensified ADR reporting is used for new and high-risk drugs. EHR mining is gaining traction with advancements in data analytics and AI. Growing emphasis on real-world evidence drives adoption of advanced reporting methods.
By Clinical Trial Phase
The market is segmented into Phase IV 40%, Phase III 25%, Phase II 15%, Phase I 10%, and Preclinical 10%, with Phase IV dominating due to mandatory post-marketing surveillance requirements and long-term safety monitoring. Phase III trials contribute significantly due to large patient populations and regulatory scrutiny. Early-phase trials require safety assessment but involve smaller datasets. Increasing drug approvals drive demand across all phases.
By End-User
End-users include Pharmaceutical Companies 65% and Hospitals 35%, with Pharmaceutical Companies leading due to regulatory obligations for adverse event reporting, risk management plans, and post-marketing surveillance. Hospitals play a critical role in reporting adverse drug reactions and supporting real-world safety data collection. Collaboration between pharma companies and healthcare institutions strengthens pharmacovigilance systems.
Buy Now & Unlock 360° Market Intelligence:
https://www.datamintelligence.com/buy-now-page?report=pharmacovigilance-market
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Regional Analysis
North America - 35% Share
North America leads with 35% share driven by stringent regulatory frameworks, high drug approval rates, and strong presence of pharmaceutical companies and CROs in the U.S. and Canada. Contract outsourcing dominates service delivery. Phase IV and spontaneous reporting are widely used. Advanced healthcare infrastructure supports market growth.
Europe - 25% Share
Europe accounts for 25% share supported by strong pharmacovigilance regulations under EMA and increasing focus on patient safety across Germany, France, and the UK. Outsourced services and EHR-based reporting are growing. Phase IV surveillance dominates. Regulatory compliance drives steady market expansion.
Asia Pacific - 20% Share
Asia Pacific holds 20% share driven by rising clinical trial activity, expanding pharmaceutical manufacturing, and improving regulatory frameworks in China, India, and Japan. Contract outsourcing is widely adopted. Phase III and Phase IV trials dominate. Increasing investment in drug safety supports market growth.
Latin America - 10% Share
Latin America records 10% share with growing adoption of pharmacovigilance services in Brazil, Mexico, and Argentina. Outsourced models dominate due to cost efficiency. Spontaneous and intensified ADR reporting lead demand. Expanding clinical research activity supports regional growth.
Middle East & Africa - 10% Share
The Middle East & Africa accounts for 10% share driven by improving healthcare regulations, rising pharmaceutical imports, and increasing awareness of drug safety, particularly in the GCC countries and South Africa. Contract outsourcing leads adoption. Phase IV surveillance and spontaneous reporting dominate. Regulatory modernization supports steady market growth.
Request for 2 Days FREE Trial Access:
https://www.datamintelligence.com/reports-subscription?prasad
✅ Competitive Landscape
✅ Technology Roadmap Analysis
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Consumer Behavior & Demand Analysis
✅ Import-Export Data Monitoring
✅ Live Market & Pricing Trends
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Germany Pharmacovigilance Market to grow at 12.43% CAGR | North America leads with 35% share | Top Companies - ArisGlobal, BioClinica, Cognizant here
News-ID: 4380579 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
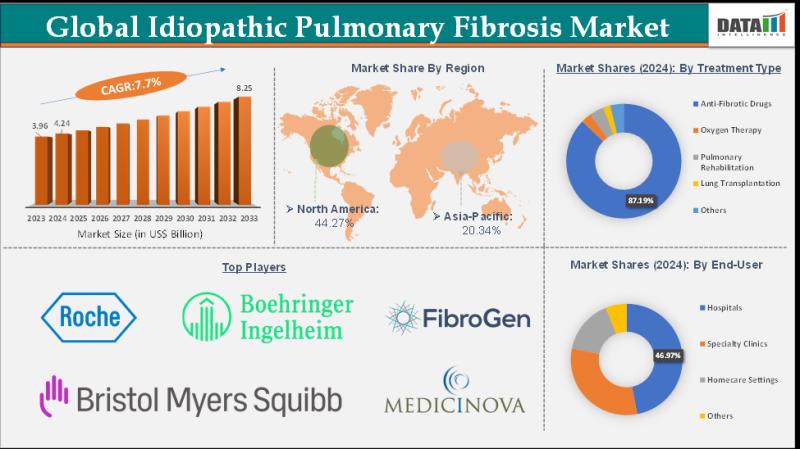
United States Idiopathic Pulmonary Fibrosis Market to hit US$ 2.47 Billion by 20 …
Leander, Texas and Tokyo, Japan - Feb.06.2026
As per DataM intelligence research report "The global idiopathic pulmonary fibrosis market size reached US$ 4.24 Billion in 2024 from US$ 3.96 Billion in 2023 and is expected to reach US$ 8.25 Billion by 2033, growing at a CAGR of 7.7% during the forecast period 2025-2033."
Growth is fueled by rising diagnosis rates and advancements in antifibrotic therapies. Pirfenidone and nintedanib dominate treatment adoption. Hospitals…

Medical Device Coatings and Methodology Market (2026-2033) | Market Expected to …
DataM Intelligence has published a new research report on "Medical Device Coatings and Methodology Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
Get a Sample PDF Of…
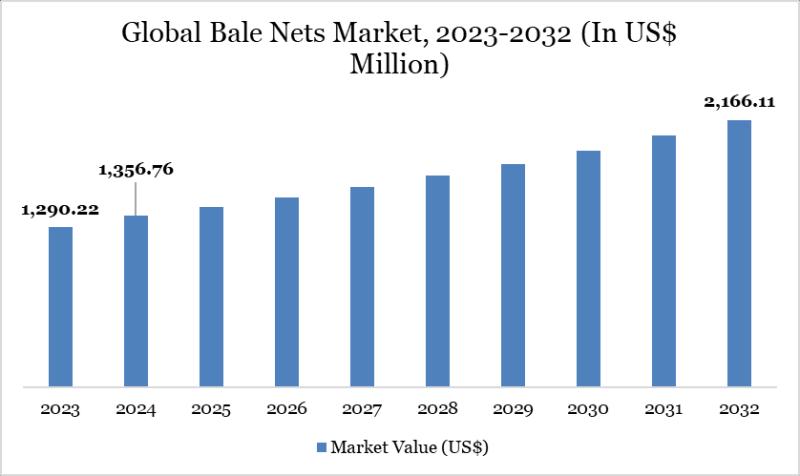
United States Bale Nets Market to Reach US$ 649.83 Billion by 2032 | North Ameri …
Leander, Texas and Tokyo, Japan - Feb.06.2026
As per DataM intelligence research report "The global bale nets market reached US$1,356.76 million in 2024 and is expected to reach US$2,166.11 billion by 2032, growing at a CAGR of 6.12% during the forecast period 2025-2032."
The market is driven by demand from agriculture and livestock sectors for efficient crop storage and handling. Polyethylene bale nets dominate usage due to durability and cost efficiency. Growing…

Understanding the Core Objectives of Logistics Automation, Market is expected to …
The global Logistics Automation Market size was accounted for USD 82.80 billion in 2025 and is predicted to increase from USD 93.40 billion in 2026 to approximately USD 260.40 billion by 2035 and is poised to grow at a compound annual growth rate (CAGR) of 12.14% during the forecast period 2026 to 2035.
The Logistics Automation Market grows due to e-commerce expansion, demand for faster deliveries, AI-driven warehouse solutions, robotics adoption,…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
