Press release
Infectious Disease Testing Product Market to Reach USD 50.22 Billion by 2033 at 7.5% CAGR; North America Leads with 38% Share, Key Players Include F. Hoffmann-La Roche, Abbott
Market OverviewThe global infectious disease testing product market reached US$ 24.41 billion in 2023, with a projected rise to US$ 26.12 billion in 2024, and is expected to reach US$ 50.22 billion by 2033, growing at a CAGR of 7.5% during the forecast period 2025-2033. The market is witnessing rapid expansion due to increasing prevalence of infectious diseases worldwide, rising demand for rapid and accurate diagnostics, and technological advancements in testing methodologies.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/infectious-disease-testing-products-market?Juli
Technological innovations are a major growth driver in the market. Advanced diagnostic techniques, such as polymerase chain reaction (PCR), next-generation sequencing (NGS), isothermal amplification, and multiplex panel testing, are enabling faster, more sensitive, and highly specific detection of pathogens. Innovations in point-of-care (POC) devices, automated testing systems, and high-throughput platforms have revolutionized clinical workflows, allowing healthcare professionals to diagnose infections in minutes to hours rather than days. These advances are particularly critical for managing highly contagious or severe infections, including COVID-19, influenza, tuberculosis, and other emerging pathogens, as early detection reduces complications, supports timely treatment, and helps prevent disease transmission.
Recent Developments:
✅ In September 2025, QIAGEN announced that its full QIAstat-Dx portfolio achieved CE-IVDR certification, including an expanded Meningitis/Encephalitis Panel. This certification reinforced the portfolio's role in clinical syndromic testing and highlighted QIAGEN's commitment to delivering high-quality infectious disease diagnostics to healthcare providers worldwide.
✅ in May 2025, SSI Diagnostica Group announced that CTK Biotech received CE-IVDR approval for three key rapid tests: OnSite Syphilis Ab Combo, OnSite Dengue Ag, and OnSite H. pylori Ag. This regulatory milestone enabled broader patient access to critical infectious disease diagnostics and expanded the group's global market footprint, strengthening its position in the competitive diagnostics industry.
Buy Now & Unlock 360° Market Intelligence:-https://www.datamintelligence.com/buy-now-page?report=infectious-disease-testing-products-market?Juli
Key Players:
F. Hoffmann-La Roche Ltd, Abbott, Cepheid, Bio-Rad Laboratories, QIAGEN, Siemens Healthcare, and Hologic, Inc.
Market Segmentation:
By product type, reagents dominate the market, accounting for approximately 45%, due to their essential role in sample preparation, amplification, and detection. Consumables hold around 30%, driven by recurring demand in high-throughput testing and routine diagnostics, while instruments represent about 25%, reflecting investment in automated and high-precision platforms.
By technology, polymerase chain reaction (PCR) is the leading technology with a 35% share, owing to its accuracy, sensitivity, and rapid detection capabilities. Sequencing and isothermal nucleic acid amplification technologies (INAAT) follow with shares of 20% and 15%, respectively, driven by applications in pathogen identification and variant detection. Mass spectrometry, chips and microarrays, in situ hybridization, and other emerging technologies collectively contribute 30%, reflecting increasing adoption in research and advanced diagnostic applications.
In terms of application, respiratory diseases testing leads with 28%, largely due to ongoing surveillance for COVID-19, influenza, and other viral infections. Tuberculosis and hepatitis testing follow with 20% and 15%, respectively, supported by government screening programs and global disease burden. Meningitis, human papillomavirus (HPV), and other applications together account for 37%, reflecting growing adoption in specialized clinical diagnostics and preventive care.
Regarding end-users, hospitals hold the largest share at 40%, driven by on-site diagnostics and patient care demands. Diagnostic laboratories contribute 30%, fueled by centralized testing services and high-throughput testing requirements. Clinics represent 20%, catering to point-of-care testing needs, while research institutes account for 10%, reflecting the use of infectious disease testing products in R&D, epidemiological studies, and translational research.
Speak to Our Analyst and Get Customization in the report as per your requirements:-https://www.datamintelligence.com/customize/infectious-disease-testing-products-market?Juli
(Single User Report: USD 4350 & One Year Database Subscription: USD 12K)
Regional Insights:
North America dominates the market, accounting for approximately 38% of the global share, due to advanced healthcare systems, high adoption of molecular diagnostics, and substantial R&D investment by key players. The U.S. and Canada lead in deploying PCR, sequencing, and point-of-care testing for both routine and outbreak management.
Europe holds around 25% of the market, supported by stringent regulatory frameworks, government-led disease screening programs, and growing demand for rapid diagnostics. Countries like Germany, the U.K., and France are major contributors, leveraging advanced laboratory networks and public health initiatives.
Market Dynamics:
Drivers: The rising prevalence of infectious diseases is a major factor fueling the growth of the infectious disease testing product market. Increased cases of HIV, tuberculosis (TB), hepatitis, COVID-19, and emerging pathogens are driving demand for accurate, rapid, and accessible diagnostics. For instance, according to WHO, in 2023, an estimated 1.3 million people acquired HIV, with a case-fatality ratio of 0.91%. The Eastern Mediterranean Region reported over 23 million cases, accounting for 3.03% of the global burden, highlighting the ongoing impact. Similarly, respiratory diseases affect over 80 million people, many of whom remain undiagnosed worldwide.
Governments, public health agencies, and global health initiatives are investing heavily in screening, surveillance, and outbreak response, which encourages innovation in molecular diagnostics, point-of-care testing, and home-based technologies. The urgency for early detection and rapid intervention continues to drive demand for infectious disease testing products.
Restraints: The high cost of advanced diagnostic equipment and tests is a significant barrier to market growth. Complex technologies, including multiplex systems, PCR machines, and next-generation sequencing (NGS) platforms, require substantial capital investment. This limits adoption, particularly in rural areas and low- and middle-income countries (LMICs) with constrained healthcare infrastructure and budgets. For example, the Xpert MTB/RIF Ultra test by Cepheid, though WHO-approved and highly accurate, faces adoption challenges due to machine costs of $17,000-$25,000 and per-test costs of $10-$20, restricting accessibility in high-burden regions.
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg?Juli
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Infectious Disease Testing Product Market to Reach USD 50.22 Billion by 2033 at 7.5% CAGR; North America Leads with 38% Share, Key Players Include F. Hoffmann-La Roche, Abbott here
News-ID: 4373662 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Regenerative Agriculture Market to Reach USD 48.14 Billion by 2032 | Cover Cropp …
The Global Regenerative Agriculture Market USD 12,634.54 million in 2024 and is projected to witness lucrative growth by reaching up to USD 48,139.24 million by 2032. The market is growing at a CAGR of 18.20% during the forecast period 2025-2032.
The Regenerative Agriculture Market is rapidly gaining traction as farmers, agribusinesses, and policymakers focus on sustainable practices that restore soil health, enhance biodiversity, and mitigate climate change. Driven by increasing consumer…

United States Neonatal Intensive Care Unit Market 2026 | Growth Drivers, Trends …
Market Size and Growth
Neonatal intensive care unit market is estimated to reach at a CAGR of 4.19% during the forecast period (2024-2031).
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/neonatal-intensive-care-unit-market?sb
Key Development:
United States: Recent Industry Developments
✅ In February 2026, VirtuAlly became one of the first in the nation to integrate experienced virtual registered nurses into a Level IV Neonatal Intensive Care Unit care team,…
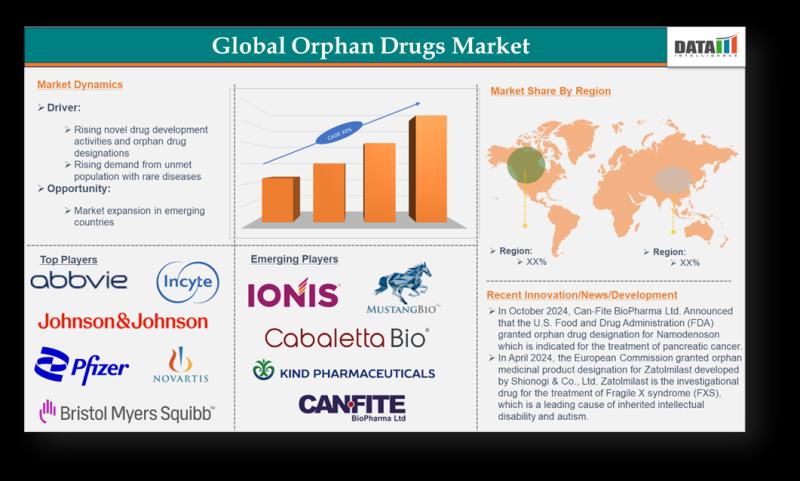
Orphan Drugs Market to Surge Beyond USD 350 Billion by 2033 | Biologics, Gene & …
The Global Orphan Drugs Market reached US$ 223.76billion in 2023 and is expected to reach US$ 486.51 billion by 2032, growing at a CAGR of 9.1% during the forecast period 2024-2032.
The Orphan Drugs Market is witnessing rapid growth as pharmaceutical companies and healthcare systems focus on developing treatments for rare and life-threatening diseases that affect small patient populations. Driven by increasing regulatory support, such as orphan drug designations, tax incentives,…
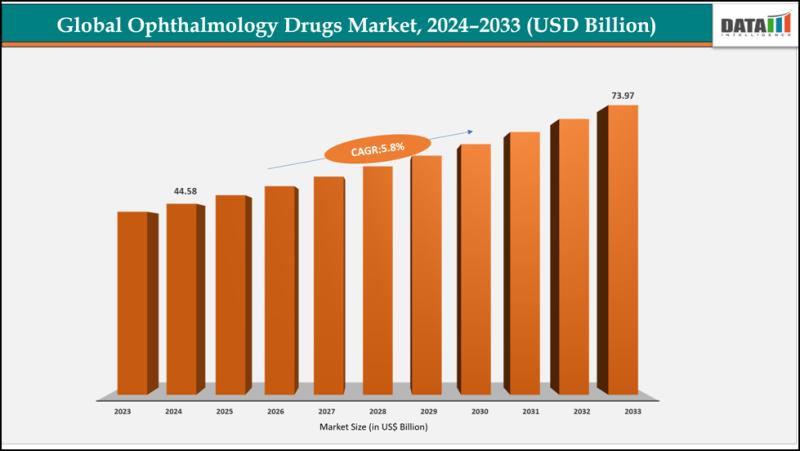
Ophthalmology Drugs Market to Reach USD 73.97 Billion by 2033 | Biologics, Anti- …
The global ophthalmology drugs market reached US$ 42.30 Billion with a rise of US$ 44.58 Billion in 2024 and is expected to reach US$ 73.97 Billion by 2033, growing at a CAGR of 5.8% during the forecast period 2025-2033.
The Ophthalmology Drugs Market is experiencing steady expansion driven by the rising prevalence of vision-related disorders such as glaucoma, dry eye syndrome, retinal diseases, and age-related macular degeneration. Increasing screen exposure, aging…
More Releases for Data
Data Catalog Market: Serving Data Consumers
Data Catalog Market size was valued at US$ 801.10 Mn. in 2022 and the total revenue is expected to grow at a CAGR of 23.2% from 2023 to 2029, reaching nearly US$ 3451.16 Mn.
Data Catalog Market Report Scope and Research Methodology
The Data Catalog Market is poised to reach a valuation of US$ 3451.16 million by 2029. A data catalog serves as an organized inventory of an organization's data assets, leveraging…
Big Data Security: Increasing Data Volume and Data Velocity
Big data security is a term used to describe the security of data that is too large or complex to be managed using traditional security methods. Big data security is a growing concern for organizations as the amount of data generated continues to increase. There are a number of challenges associated with securing big data, including the need to store and process data in a secure manner, the need to…
HOW TO TRANSFORM BIG DATA TO SMART DATA USING DATA ENGINEERING?
We are at the cross-roads of a universe that is composed of actors, entities and use-cases; along with the associated data relationships across zillions of business scenarios. Organizations must derive the most out of data, and modern AI platforms can help businesses in this direction. These help ideally turn Big Data into plug-and-play pieces of information that are being widely known as Smart Data.
Specialized components backed up by AI and…
Test Data Management (TDM) Market - test data profiling, test data planning, tes …
The report categorizes the global Test Data Management (TDM) market by top players/brands, region, type, end user, market status, competition landscape, market share, growth rate, future trends, market drivers, opportunities and challenges, sales channels and distributors.
This report studies the global market size of Test Data Management (TDM) in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption…
Data Prep Market Report 2018: Segmentation by Platform (Self-Service Data Prep, …
Global Data Prep market research report provides company profile for Alteryx, Inc. (U.S.), Informatica (U.S.), International Business Corporation (U.S.), TIBCO Software, Inc. (U.S.), Microsoft Corporation (U.S.), SAS Institute (U.S.), Datawatch Corporation (U.S.), Tableau Software, Inc. (U.S.) and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical analysis 2012 to 2017, market forecast 2018 to 2025 in terms of volume, revenue, YOY…
Long Term Data Retention Solutions Market - The Increasing Demand For Big Data W …
Data retention is a technique to store the database of the organization for the future. An organization may retain data for several different reasons. One of the reasons is to act in accordance with state and federal regulations, i.e. information that may be considered old or irrelevant for internal use may need to be retained to comply with the laws of a particular jurisdiction or industry. Another reason is to…
