Press release
Clinical Trial Management System (CTMS) Market Forecast to Reach USD 5.21 Billion by 2033, Driven by Growing Complexity of Clinical Trials and Pharma-Biotech Adoption
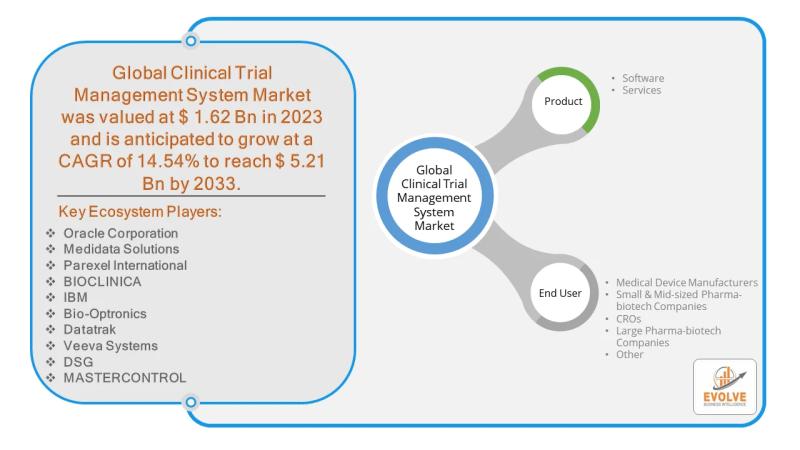
The global Clinical Trial Management System (CTMS) market is experiencing significant growth, with a projected value of $5.21 billion by 2033, up from $1.62 billion in 2023, and a robust compound annual growth rate (CAGR) of 14.54%. This expansion is primarily driven by the increasing complexity of clinical trials and the rising adoption of advanced technologies. Among the key players, large pharma-biotech companies are in a prime position to capitalize on this market due to their extensive involvement in large-scale clinical trials.Download the full report now to discover market trends, opportunities, and strategies for success.
https://evolvebi.com/report/clinical-trial-management-system-market-analysis/
Problems Faced by Large Pharma-biotech Companies
Despite the promising market, large pharma-biotech companies face several challenges in managing clinical trials effectively:
• Operational Inefficiencies: Managing multiple trials simultaneously, often across different geographic locations, leads to logistical complexities and data fragmentation. This can slow down the trial process and increase costs.
• Data Security and Privacy Concerns: Clinical trials involve highly sensitive patient data. Ensuring the security and privacy of this information is paramount and a constant challenge, particularly with the rise of remote patient monitoring and decentralized trials.
• Regulatory Compliance: Adhering to diverse and evolving regulatory standards across different countries can be a significant administrative burden and a source of potential delays and non-compliance.
• Supply Chain Disruptions: The reliance on global supply chains for trial materials and equipment makes them vulnerable to geopolitical issues and trade policies, such as tariffs.
________________________________________
A Proposed Solution: Integrated CTMS with Emerging Technologies
A comprehensive solution for large pharma-biotech companies involves the adoption of an integrated CTMS that leverages emerging technologies to address the aforementioned challenges. This solution would:
• Enhance Operational Efficiency: An integrated system would streamline the planning, tracking, and administrative aspects of clinical trials, providing a centralized platform for all stakeholders. This would improve communication and reduce the time and resources spent on manual data entry and reconciliation.
• Strengthen Data Security: By incorporating blockchain technology, the CTMS can create an immutable, transparent, and secure record of all clinical trial data, ensuring data integrity and enhanced patient privacy.
• Ensure Regulatory Adherence: A sophisticated CTMS can be configured to automate compliance checks and provide real-time reporting, helping companies stay ahead of regulatory requirements and reduce the risk of non-compliance.
• Optimize Supply Chain Logistics: The integration of AI and machine learning can forecast demand for trial supplies and equipment, mitigating the impact of supply chain bottlenecks and delays.
Download the full report now to discover market trends, opportunities, and strategies for success.
https://evolvebi.com/report/clinical-trial-management-system-market-analysis/
US Tariff Implications on the Clinical Trial Management System Market
While clinical trial lots and pre-commercial R&D supplies are generally exempt from tariffs under the Harmonized Tariff Schedule Code 9817.85 (prototypes), the CTMS market is not entirely immune to the broader effects of US tariffs. The key implications include:
• Indirect Cost Increases: Tariffs on medical supplies, lab equipment, and other ancillary products used in clinical trials can increase overall operational costs. These costs can be passed on to hospitals and research sites, ultimately raising the total budget for a trial.
• Supply Chain Disruption: Tariffs and trade policies can disrupt the supply of essential trial materials, leading to delays. This can be particularly impactful for small and mid-sized biotechs with limited contingency resources, but large pharma companies must also navigate this increased volatility.
• Shift in Trial Locations: While unlikely to completely deter US-based trials, rising costs due to tariffs could make other countries with lower operational costs, such as India and South Korea, more attractive for sponsors, especially for certain trial phases.
• Focus on Domestic Sourcing: Tariffs may encourage a strategic shift toward US-centric or regional trial execution and supply chain sourcing to mitigate exposure to international trade risks. This could lead to an increased demand for domestic CTMS solutions and related services.
In conclusion, large pharma-biotech companies have a significant opportunity to drive the growth of the CTMS market by adopting advanced, integrated solutions that address existing problems. While US tariffs pose some challenges, particularly in an indirect manner, a well-planned and technologically advanced CTMS can help mitigate these risks and ensure the continued success of clinical trials.
Download the full report now to discover market trends, opportunities, and strategies for success.
https://evolvebi.com/report/clinical-trial-management-system-market-analysis/
To understand further and explore opportunities in the Clinical Trial Management System Market or any related industry, please share your queries/concerns at info@evolvebi.com.
Evolve Business Intelligence
C-218, 2nd floor, M-Cube
Gujarat 396191
India
Email: sales@evolvebi.com
Website: https://evolvebi.com/
Evolve Business Intelligence is a market research, business intelligence, and advisory firm providing
innovative solutions to challenging pain points of a business. Our market research reports include data
useful to micro, small, medium, and large-scale enterprises. We provide solutions ranging from mere
data collection to business advisory.
Evolve Business Intelligence is built on account of technology advancement providing highly accurate
data through our in-house AI-modelled data analysis and forecast tool - EvolveBI. This tool tracks real-
time data including, quarter performance, annual performance, and recent developments from
fortune's global 2000 companies.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management System (CTMS) Market Forecast to Reach USD 5.21 Billion by 2033, Driven by Growing Complexity of Clinical Trials and Pharma-Biotech Adoption here
News-ID: 4372055 • Views: …
More Releases from Evolve Business Intelligence
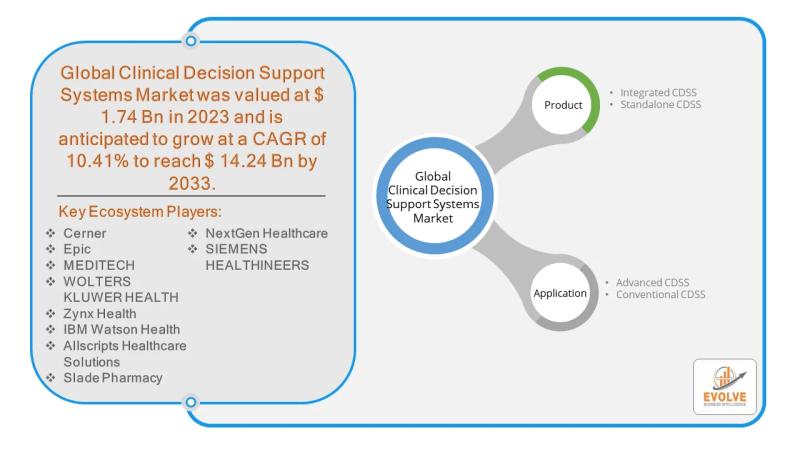
Clinical Decision Support Systems Market Forecast to Reach USD 14.24 Billion by …
The Clinical Decision Support Systems (CDSS) Market, a pivotal segment of the broader healthcare IT industry, is poised for significant growth. While the market for conventional CDSS, such as those integrated into Electronic Health Records (EHRs) for basic drug alerts, is well-established, Advanced Clinical Decision Support Systems present a new frontier of opportunity. These next-generation systems leverage artificial intelligence (AI), machine learning, and big data to offer more sophisticated insights,…
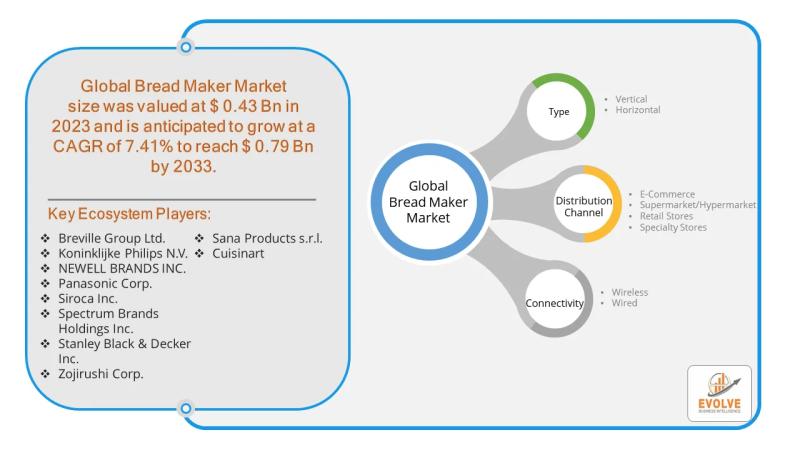
Bread Maker Market Forecast to Reach USD 0.79 Billion by 2033, Driven by Rising …
The market for bread makers is experiencing a resurgence, driven by a growing interest in home baking, health-conscious consumers, and a desire for convenience. While once seen as a niche kitchen appliance, the modern bread maker has evolved, offering a streamlined process for creating fresh, artisanal bread at home. This shift presents a high-opportunity landscape, especially within the e-commerce sector, which can leverage digital platforms to directly reach and engage…
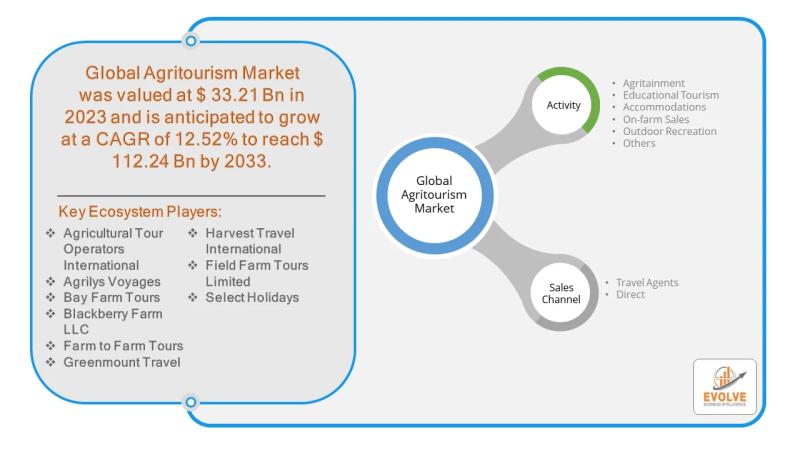
Agritourism Market Forecast to Reach USD 112.24 Billion by 2033, Driven by Risin …
The agritourism market is experiencing a significant surge, transforming rural landscapes and offering new economic opportunities for farmers. Agritourism, which blends agriculture with tourism, is projected to reach a market size of USD 112.24 billion by 2033, a substantial increase from USD 33.21 billion in 2023, with a Compound Annual Growth Rate (CAGR) of 12.52%. While activities like agritainment and educational tours are dominant segments, on-farm sales present a high-opportunity…
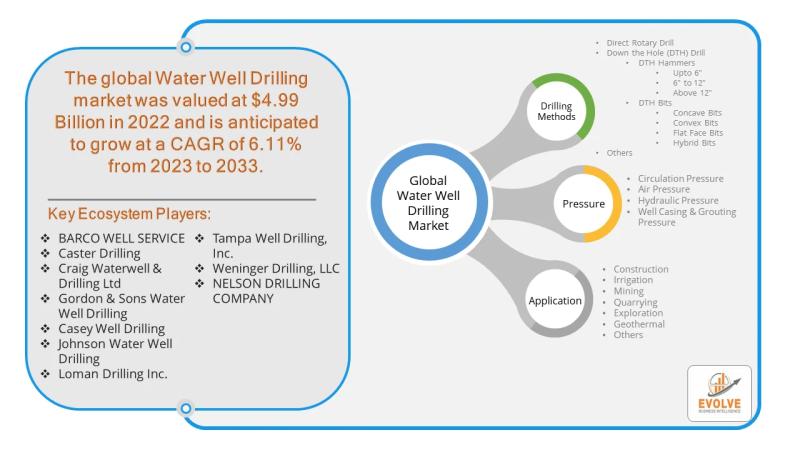
Water Well Drilling Market Forecast to Reach USD 21.92 Billion by 2028, Driven b …
The global Water Well Drilling Market, valued at $4.99 Billion in 2022, is projected to grow at a CAGR of 6.11% from 2023 to 2033, reaching an estimated $21.92 billion by 2028. This growth is fueled by increasing demand for freshwater, population growth, and technological advancements. Among these advancements, the use of hydraulic pressure is emerging as a significant opportunity, offering efficiency and control in drilling operations.
Download the full report…
More Releases for CTMS
Key Trends Shaping the Future Clinical Trial Management System CTMS Market From …
What Is the Estimated Market Size and Growth Rate for the Clinical Trial Management System CTMS Market?
The clinical trial management system (CTMS) market has experienced rapid growth in recent years. It is expected to grow from $1.41 billion in 2024 to $1.61 billion in 2025, with a CAGR of 14.2%. Growth factors include increasing clinical trial complexity, a rise in global clinical trials, regulatory compliance demands, an increasing focus on…
Clinical Trial Management System (CTMS) Market: Growth, Trends, Opportunities, a …
Introduction
A Clinical Trial Management System (CTMS) is an integrated software platform designed to streamline and manage the planning, execution, and monitoring of clinical trials. It helps healthcare organizations, pharmaceutical companies, research institutions, and contract research organizations (CROs) efficiently oversee the complex and data-driven processes of clinical trials. The CTMS enables management of trial activities such as patient recruitment, data collection, regulatory compliance, budgeting, and reporting.
Clinical trials are a critical component…
Clinical Trial Management System (CTMS) Market: Growth, Opportunities, and Chall …
Introduction
The Clinical Trial Management System (CTMS) market is a crucial segment of the global healthcare and pharmaceutical industries, which supports the efficient and accurate management of clinical trials. CTMS refers to software systems used by pharmaceutical, biotechnology, and contract research organizations (CROs) to streamline the planning, tracking, and management of clinical trials. These systems provide centralized data management for clinical trial activities, which helps improve the speed, quality, and compliance…
Clinical Trial Management System (CTMS) Global Market Report 2024 - Clinical Tri …
"The Business Research Company recently released a comprehensive report on the Global Clinical Trial Management System (CTMS) Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The…
Clinical Trial Management System (CTMS) Market Scope & Future Opportunities Till …
An exclusive Clinical Trial Management System (CTMS) Market research report has been fabricated through the in depth analysis of the market dynamics across five regions including North America, Europe, South America, Asia-Pacific, Middle East and Africa. The segmentation of the market by components, end users, and region was done based on the thorough market analysis and validation through extensive primary inputs from industry experts (key opinion leaders of companies, and…
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…