Press release
Pharmacovigilance and Drug Safety Software Market 2025-2032: AI-Driven Automation and Regulatory Compliance Fuel Growth
The pharmacovigilance and drug safety software market reached approximately US$ 3.66 billion in 2024 and is expected to reach around US$ 8.48 billion by 2032, growing at a CAGR of about 12.2% during the forecast period 2025-2032. Market growth is driven by increasing regulatory requirements for adverse event reporting, rising volume of safety data from clinical trials and post-marketing sources, and growing adoption of automated software solutions to improve signal detection, case management, and compliance. Integration of artificial intelligence, machine learning, and real-world evidence analytics is enhancing efficiency, accuracy, and proactive risk management in pharmacovigilance operations.North America held the largest market share due to stringent regulatory frameworks, high pharmaceutical and biotech activity, and early adoption of advanced drug safety software solutions. Europe followed with strong emphasis on drug safety and well-established reporting systems, while Asia-Pacific is expected to witness the fastest growth driven by expanding pharmaceutical manufacturing and clinical trial activity, increasing regulatory focus on drug safety, and rising investments in digital health technologies across emerging economies.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/pharmacovigilance-and-drug-safety-software-market?sai-v
The pharmacovigilance and drug safety software market refers to the global industry focused on digital platforms and tools used to monitor, analyze, and manage adverse drug events and ensure regulatory compliance and patient safety.
Key Developments
✅ January 2026: In North America, life sciences companies accelerated integration of AI-driven safety signal detection and predictive analytics into pharmacovigilance software platforms to enhance early identification of adverse drug reactions and improve risk management workflows.
✅ January 2026: In Europe, regulatory agencies updated compliance guidelines to support real-time safety reporting standards and electronic submissions, prompting software developers to enhance automation and regulatory interoperability across case management systems.
✅ December 2025: In Asia-Pacific, increased adoption of cloud-native drug safety platforms enabled pharmaceutical sponsors and contract safety service providers to scale global case intake, processing, and reporting with improved data security and performance.
✅ December 2025: Globally, advancements in natural language processing (NLP) and machine learning strengthened automated literature screening, social media safety monitoring, and unstructured data ingestion into drug safety software suites.
✅ November 2025: In Latin America, expansion of national adverse event reporting initiatives encouraged broader deployment of integrated pharmacovigilance case management and analytics tools to support post-marketing surveillance.
✅ October 2025: Worldwide, enhancements in workflow automation and API integration improved interoperability between drug safety software, electronic health records (EHRs), and regulatory submission systems, streamlining end-to-end safety operations.
Mergers & Acquisitions
✅ January 2026: In the United States, a leading life sciences technology provider acquired an AI-based pharmacovigilance analytics company to expand automated signal detection and predictive safety capabilities within its drug safety software portfolio.
✅ December 2025: In Europe, a major regulatory compliance software firm completed the acquisition of a cloud-native drug safety platform developer to broaden its end-to-end pharmacovigilance and safety reporting solutions.
✅ November 2025: In Asia-Pacific, a regional technology and services company acquired a natural language processing tools provider to strengthen its drug safety software offerings with advanced literature and social media monitoring features.
Key Players
Ennov Solutions Inc. | Oracle Corporation | ArisGlobal LLC | EXTEDO | Clinevo Technologies | IQVIA | AB Cube | ICON Plc | Paraxel International Corporation | Others
Key Highlights
Ennov Solutions Inc. holds 18.7% share, driven by its specialized regulatory information management (RIM) solutions, strong compliance capabilities, and growing adoption among pharmaceutical and biotech companies.
Oracle Corporation holds 16.9% share, supported by its enterprise-grade cloud platforms, life sciences data management solutions, and global customer base.
ArisGlobal LLC holds 14.8% share, leveraging its integrated life sciences software suite, pharmacovigilance expertise, and regulatory compliance tools.
EXTEDO holds 12.6% share, driven by its regulatory affairs and submission management solutions, strong presence in Europe, and long-standing regulatory expertise.
Clinevo Technologies holds 9.4% share, supported by cloud-native regulatory platforms and focus on user-friendly compliance workflows.
IQVIA holds 8.7% share, leveraging its clinical and regulatory data assets, technology-enabled services, and global regulatory consulting capabilities.
AB Cube holds 7.3% share, driven by its agile regulatory software solutions and strong adoption among mid-sized pharmaceutical companies.
ICON Plc holds 6.1% share, supported by its CRO expertise, regulatory support services, and integration across clinical development workflows.
Paraxel International Corporation holds 4.2% share, leveraging its global CRO footprint, regulatory consulting services, and strong relationships with regulators.
Others account for 0.2% share, comprising niche regulatory technology vendors and emerging compliance solution providers.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=pharmacovigilance-and-drug-safety-software-market?sai-v
Market Drivers
- Increasing global regulatory scrutiny and stringent compliance requirements for adverse event reporting and drug safety monitoring.
- Rising volume of adverse drug reactions and post-marketing safety data driving demand for automated pharmacovigilance software.
- Growing adoption of AI, machine learning, and natural language processing to improve case processing efficiency and signal detection accuracy.
- Expansion of cloud-based and SaaS drug safety platforms enabling scalable and cost-effective global operations.
- Increasing integration of real-world data, electronic health records, and digital health sources into safety surveillance workflows.
Industry Developments
- Launch of fully integrated pharmacovigilance and drug safety software suites covering case intake, signal management, risk assessment, and regulatory submissions.
- Enhanced AI-driven automation for adverse event coding, duplicate detection, and workflow management.
- Growing adoption of cloud-native and modular platforms supporting multi-country regulatory compliance.
- Development of real-time and mobile adverse event reporting tools to accelerate safety data capture.
- Strategic partnerships between software vendors, pharmaceutical companies, and CROs to expand functionality and global reach.
Regional Insights
North America - 40% share: "Driven by stringent FDA safety regulations, high drug approval volumes, advanced healthcare IT infrastructure, and strong adoption of pharmacovigilance software."
Europe - 28% share: "Supported by harmonized EU pharmacovigilance standards, strong regulatory oversight, and widespread use of cloud-based safety platforms."
Asia Pacific - 22% share: "Fueled by expanding pharmaceutical manufacturing, growing clinical trial activity, increasing regulatory alignment, and rising digital transformation."
Latin America - 6% share: "Driven by increased outsourcing of pharmacovigilance activities and adoption of cost-effective cloud-based drug safety solutions."
Middle East & Africa - 4% share: "Supported by strengthening regulatory frameworks, expanding healthcare IT infrastructure, and growing focus on patient safety monitoring."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/pharmacovigilance-and-drug-safety-software-market?sai-v
Key Segments
By Service Provider
Contract outsourcing dominates the market, driven by increasing dependence on specialized pharmacovigilance service providers to manage complex safety data, ensure regulatory compliance, and optimize operational efficiency. In-house services hold a significant share, particularly among large pharmaceutical companies that prefer maintaining internal pharmacovigilance teams for greater control over drug safety processes.
By Type of Reporting
Spontaneous reporting represents the largest segment, supported by its widespread use as the primary mechanism for adverse drug reaction (ADR) detection. Intensified ADR reporting accounts for a notable share, driven by heightened regulatory scrutiny and enhanced safety monitoring requirements for high-risk medications. Cohort event monitoring is gaining traction due to its structured approach to assessing drug safety within defined patient populations. Targeted spontaneous reporting is expanding steadily, supported by its effectiveness in monitoring specific drugs or therapeutic classes. EHR mining represents an emerging segment, driven by increasing adoption of electronic health records and advanced analytics for real-world safety signal detection.
By Clinical Trial Phase
Phase IV dominates the market, driven by stringent post-marketing surveillance requirements and ongoing safety evaluation of approved drugs. Phase III holds a substantial share due to large-scale patient enrollment and extensive safety data generation prior to regulatory approval. Phase II represents a significant segment, supported by dose-ranging and efficacy assessment studies. Phase I accounts for a smaller share, focused on early-stage safety and tolerability evaluation. Preclinical pharmacovigilance contributes modestly, primarily supporting early toxicology and risk assessment activities.
By End-User
Pharmaceutical companies represent the largest end-user segment, driven by regulatory obligations to monitor drug safety throughout the product lifecycle. Hospitals hold a significant share, supported by their role in direct patient care, ADR identification, and real-world safety reporting.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance and Drug Safety Software Market 2025-2032: AI-Driven Automation and Regulatory Compliance Fuel Growth here
News-ID: 4359852 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

U.S. Alzheimer Drugs Market Set for Explosive Growth to USD 8.84 Billion by 2031 …
Leander Texas -
The Alzheimer Drugs Market reached US$ 4.46 billion in 2023 and is expected to reach US$ 18.33 billion by 2031, growing at a CAGR of 19.4% during the forecast period 2024-2031.
The Alzheimer's drugs market growth is driven by key US-Japan collaborations and approvals, including the JCR Pharma-Acumen Pharmaceuticals partnership to develop a novel blood-brain barrier Alzheimer therapy and expanded use of Eisai/Biogen's lecanemab with new subcutaneous application and…

Micro Nuclear Reactors (MNRs) Market to Reach US$ 4,865.85 Million by 2032 at 18 …
The Micro Nuclear Reactors (MNRs) Market reached US$ 1,434.55 million in 2024 and is projected to reach US$ 4,865.85 million by 2032, growing at a CAGR of 18.26 percent during the forecast period 2025 to 2032.
Market growth is driven by increasing demand for reliable, low-carbon energy solutions, supportive government policies promoting clean power generation, and the need for decentralized power in remote and industrial applications. Micro nuclear reactors offer scalable,…
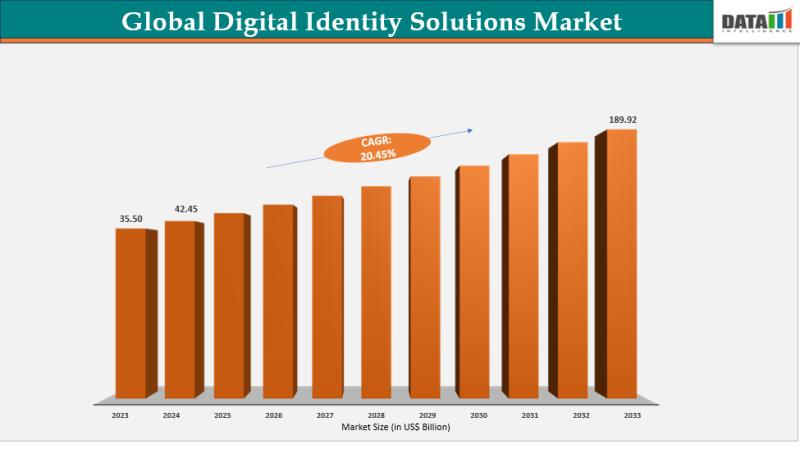
Digital Identity Solutions Market Set for Explosive Growth to US$189.92 Billion …
The Global Digital Identity Solutions Market reached US$35.50 billion in 2023, with a rise to US$42.45 billion in 2024, and is expected to reach US$189.92 billion by 2033, growing at a CAGR of 20.45% during the forecast period 2025-2033.
Market growth is driven by escalating cybersecurity threats, surging demand for secure authentication in remote work and e-commerce, and widespread adoption of biometric and blockchain-based verification. Advancements in AI-powered fraud detection, expanding…

Iron Ore Mining Market Set for Strong Growth to USD 620.7 Billion by 2031, Led b …
Leander Texas -
Iron Ore Mining Market reached US$ 330.2 billion in 2022 and is expected to reach US$ 620.7 billion by 2031, growing with a CAGR of 8.2% during the forecast period 2024-2031.
The Iron Ore Mining Market's strong growth is boosted by rising infrastructure and steel demand in the U.S. and Japan, coupled with strategic developments like Japanese firms acquiring stakes in global iron ore assets and collaborations with U.S.…
More Releases for America
Stabilit America Highlights Applications of Fiberglass Roof Panels with Stabilit …
Roofing materials are very important in the realm of modern construction, as they should be long lasting, economical and attractive. Fiberglass roof panels are a few of the numerous choices among several alternatives that have received a reputation of being versatile, long life, and adaptable in various sectors. They are favored by the architects, contractors, and property developers due to their lightweight construction, resistance to weather factors, and the ease…
Deodorants Market Report by Region (North America, EMEA, Latin America, Asia)
2025 - Pristine Market Insights, a leading market research firm, announced the release of its latest and comprehensive market research report on Deodorants market. The report spans over 500 pages and delivers 10-year market forecast in US dollars (or custom currencies upon request). It provides in-depth analysis of market dynamics (drivers, opportunities, restraints), PESTLE insights, latest industry trends, and demand factors. The report includes segmented market value, share (%), compound…
Sequestrant Market Report by Region (North America, EMEA, Latin America, Asia)
2025 - Pristine Market Insights, a leading market research firm, announced the release of its latest and comprehensive market research report on Sequestrant market. The report spans over 500 pages and delivers 10-year market forecast in US dollars (or custom currencies upon request). It provides in-depth analysis of market dynamics (drivers, opportunities, restraints), PESTLE insights, latest industry trends, and demand factors. The report includes segmented market value, share (%), compound…
Buttermilk Market Study by Region (North America, Latin America, Europe, Asia, M …
2025 - Pristine Market Insights, a leading market research firm, announced the release of its latest and comprehensive market research report on Buttermilk market. The report spans over 500 pages and delivers 10-year market forecast in US dollars (or custom currencies upon request). It provides in-depth analysis of market dynamics (drivers, opportunities, restraints), PESTLE insights, latest industry trends, and demand factors. The report includes segmented market value, share (%),…
Textiles Market Analysis Report, Regional Outlook - Europe, North America, South …
Adroit Market Research has announced the addition of the “Global Textiles Market Size Status and Forecast 2025”, The report classifies the global Textiles in a precise manner to offer detailed insights about the aspects responsible for augmenting as well as restraining market growth.
This report studies the global Textiles Speaker market, analyzes and researches the Textiles Speaker development status and forecast in Europe, North America, Central America, South America, Asia Pacific…
Global Gaucher Disease Market 2018 Covering North America, South America, Europe
Gaucher Disease Market
Summary
The Global Gaucher Disease Market is defined by the presence of some of the leading competitors operating in the market, including the well-established players and new entrants, and the suppliers, vendors, and distributors. The key players are continuously focusing on expanding their geographic reach and broadening their customer base, in order to expand their product portfolio and come up with new advancements.
Gaucher Disease market size to maintain the average annual growth…
