Press release
Viral Inactivation Market to Reach US$ 3.18 Billion by 2032 at 12.4% CAGR; North America Leads with 42% Share | Key Players Charles River Laboratories, Danaher, Merck KGaA
The Viral Inactivation Market reached US$ 1.98 billion in 2024 and is expected to reach US$ 3.18 billion by 2032, growing at a CAGR of 12.4% during the forecast period 2025-2032. The market is expanding steadily as viral inactivation has become a critical step in ensuring the safety of biologics, vaccines, blood products, and plasma-derived therapies.Growth is driven by rising production of biologics and biosimilars, increasing demand for blood safety, and stringent regulatory requirements for viral clearance in pharmaceutical manufacturing. Adoption of advanced viral inactivation methods such as solvent/detergent treatment, low-pH inactivation, heat treatment, and nanofiltration is improving process reliability and compliance. While challenges related to process validation and cost remain, ongoing innovation and expanding biopharmaceutical pipelines continue to support sustained market growth.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/viral-inactivation-market?sai-v
Viral Inactivation Market is the global market for technologies and processes used to eliminate or reduce viral contaminants in biopharmaceuticals, blood products, and medical devices to ensure product safety.
Key Developments
✅ January 2026: In the United States, biopharmaceutical manufacturers increased adoption of advanced viral inactivation technologies such as solvent/detergent treatment, low-pH inactivation, and UV-based methods to enhance safety in plasma-derived products, monoclonal antibodies, and cell & gene therapies.
✅ January 2026: In Europe, regulatory emphasis on viral safety validation strengthened demand for integrated viral inactivation steps within biologics manufacturing workflows, particularly for vaccines and recombinant protein therapeutics.
✅ January 2026: In Japan, pharmaceutical companies expanded the use of single-use systems incorporating validated viral inactivation processes to improve manufacturing flexibility and contamination control.
✅ December 2025: In the United States, growth in plasma fractionation and contract manufacturing activities drove investments in scalable viral inactivation solutions aligned with FDA and global regulatory standards.
✅ December 2025: In Asia-Pacific, expanding biologics and biosimilars production capacity supported wider implementation of robust viral clearance and inactivation strategies across upstream and downstream processing.
✅ November 2025: In Europe, increasing production of blood products and advanced therapies accelerated adoption of automated viral inactivation equipment to improve consistency and compliance.
Mergers & Acquisitions
✅ January 2026: In the United States, a life sciences technology provider acquired a viral inactivation and pathogen reduction technology firm to expand its bioprocessing safety portfolio.
✅ December 2025: In Europe, a bioprocess solutions company completed the acquisition of a viral clearance validation specialist to strengthen regulatory support services for biologics manufacturers.
✅ December 2025: In Japan, a pharmaceutical equipment manufacturer acquired a niche supplier of viral inactivation components to enhance end-to-end biomanufacturing offerings.
✅ October 2025: In Asia-Pacific, a contract development and manufacturing organization (CDMO) acquired a regional bioprocess technology company to integrate viral inactivation capabilities into its biologics production services.
Key Players
Charles River Laboratories International, Inc. | Clean Cells Inc. | Danaher Corporation | Cerus Corporation | Parker Hannifin Corporation | Merck KGaA | Rad Source Technologies, Inc. | Sartorius AG | SGS SA | Wuxi Pharmatech (Cayman) Inc. | Others
Key Highlights
Charles River Laboratories International, Inc. holds 19.6% share, driven by its strong leadership in biologics testing services, cell and gene therapy support, and broad adoption across pharmaceutical and biotechnology companies.
Danaher Corporation holds 17.3% share, supported by its life sciences instrumentation, bioprocessing technologies, and continuous innovation across regulated laboratory environments.
Merck KGaA holds 15.1% share, leveraging its extensive portfolio of bioprocessing reagents, filtration systems, and cell processing solutions.
Sartorius AG holds 12.8% share, driven by its advanced cell processing, sterility testing, and biopharmaceutical manufacturing solutions.
Wuxi Pharmatech (Cayman) Inc. holds 11.2% share, supported by strong CRO/CDMO capabilities and growing demand from global biologics developers.
SGS SA holds 8.4% share, focused on quality assurance, validation, and testing services across pharmaceutical and biotechnology sectors.
Cerus Corporation holds 6.1% share, driven by pathogen reduction technologies and blood safety solutions.
Parker Hannifin Corporation holds 4.7% share, leveraging fluid handling, filtration, and contamination control technologies used in life sciences applications.
Rad Source Technologies, Inc. holds 2.9% share, supported by non-isotopic irradiation systems for sterilization and pathogen inactivation.
Clean Cells Inc. holds 1.5% share, focused on niche cell banking, viral safety testing, and biologics support services.
Others account for 0.4% share, comprising regional and specialized service providers in biopharmaceutical testing and safety solutions.
Buy Now & Unlock 360° Market Intelligence: https://www.datamintelligence.com/buy-now-page?report=viral-inactivation-market?sai-v
Market Drivers
- Rising demand for viral inactivation technologies due to increasing prevalence of viral infections, pandemics, and stringent biosafety requirements.
- Growing use of inactivation methods in vaccine development, blood safety, and biopharmaceutical manufacturing.
- Stringent regulatory standards for sterilization, decontamination, and pathogen control in healthcare and laboratory settings.
- Expansion of molecular diagnostics and research requiring safe handling of viral samples.
- Advancements in inactivation technologies improving efficacy, speed, and scalability.
Industry Developments
- Development of advanced chemical, heat, and UV-based viral inactivation solutions for diverse applications.
- Integration of viral inactivation protocols in bioprocessing workflows for vaccines, biologics, and therapeutic products.
- Strategic collaborations between biotech firms, diagnostic companies, and research institutions to enhance viral safety solutions.
- Launch of automated and closed-system inactivation platforms for high-throughput laboratories.
- Increasing focus on standardized, validated inactivation methods for regulatory compliance and quality assurance.
Regional Insights
North America - 42% share: "Driven by strong healthcare infrastructure, high biopharmaceutical production, and stringent safety regulations for viral handling."
Europe - 28% share: "Supported by robust regulatory frameworks, advanced research activities, and rising adoption of validated inactivation technologies."
Asia Pacific - 24% share: "Fueled by expanding biotech and vaccine manufacturing sectors, rising research investments, and growing demand for safe viral workflows."
Latin America - 4% share: "Driven by increasing molecular diagnostics adoption, improving healthcare services, and gradual implementation of inactivation standards."
Middle East & Africa - 2% share: "Supported by emerging laboratory infrastructure, growing focus on biosafety, and increasing public health initiatives."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/viral-inactivation-market?sai-v
Key Segments
By Method
The solvent detergent method holds a significant share, driven by its proven effectiveness in inactivating lipid-enveloped viruses and its widespread adoption in biopharmaceutical manufacturing. Pasteurization is widely used, supported by its reliability and long history in ensuring viral safety of plasma-derived products. Other methods are gaining traction as manufacturers adopt advanced and complementary viral inactivation techniques to enhance product safety.
By Product
Kits and reagents represent a major segment, supported by recurring demand in routine viral safety testing and inactivation workflows. Services are witnessing steady growth, driven by increasing outsourcing of viral clearance and validation studies to specialized providers. Viral inactivation systems and accessories are expanding in adoption as biopharmaceutical companies invest in integrated and automated solutions.
By Application
Vaccines and therapeutics account for the largest application segment, driven by rising global vaccine production and biologics development. Blood and blood products represent a critical segment due to strict regulatory requirements for viral safety. Cellular and gene therapy products are experiencing rapid growth, supported by advancements in advanced therapy medicinal products. Tissues and tissue products, along with stem cell products, continue to see growing adoption as regenerative medicine expands.
By End-Users
Pharmaceutical and biotechnology companies dominate the market, driven by extensive biologics manufacturing and regulatory compliance needs. Contract research organizations are gaining traction due to increasing outsourcing of viral inactivation and safety studies. Other end-users contribute through academic, research, and specialized manufacturing activities.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Viral Inactivation Market to Reach US$ 3.18 Billion by 2032 at 12.4% CAGR; North America Leads with 42% Share | Key Players Charles River Laboratories, Danaher, Merck KGaA here
News-ID: 4353573 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Live Cell Imaging Market to Reach US$ 6.8 Billion by 2032 at 11.2% CAGR; North A …
The Live Cell Imaging Market reached US$ 2.9 billion in 2024 and is expected to reach US$ 6.8 billion by 2032, growing at a CAGR of 11.2% during the forecast period 2025-2032. The market is expanding steadily as live cell imaging becomes a critical technique for observing cellular dynamics, structure, and behavior in real time across life sciences and biomedical research.
Growth is driven by increasing adoption in drug discovery, cancer…
United States Circuit Breaker Market to Reach US$ 33.34 billion by 2032 | CAGR 5 …
Circuit Breaker Market reached US$ 21.38 billion in 2024 and is expected to reach US$ 33.34 billion by 2032, growing with a CAGR of 5.71% during the forecast period 2025-2032.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/circuit-breaker-market?kb
United States: Recent Industry Developments
✅ January 2026: Eaton launched smart circuit breakers with IoT integration to improve real-time electrical system monitoring and fault detection.
✅ December 2025:…
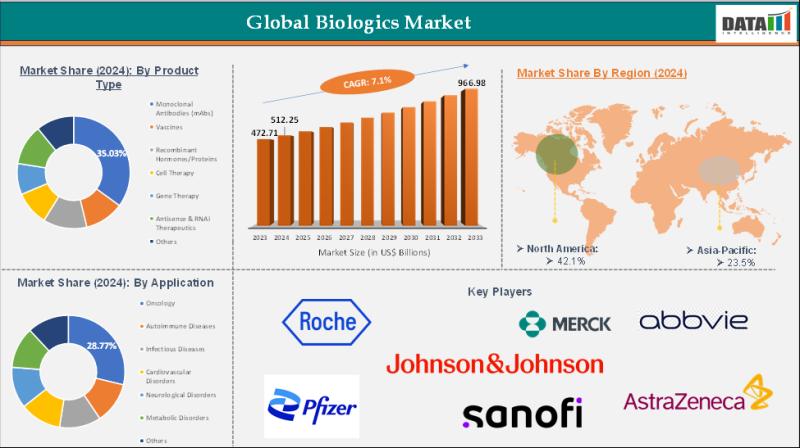
United States Biologics Market to Reach US$ 290.09 Billion by 2033 | Top Compani …
Leander, Texas and Tokyo, Japan - Jan.19.2026
As per DataM intelligence research report "The global biologics market reached US$ 472.71 billion in 2023, with a rise of US$ 512.25 billion in 2024, and is expected to reach US$ 966.98 billion by 2033, growing at a CAGR of 7.1% during the forecast period 2025-2033."
The biologics market is expanding rapidly as advanced therapies, including monoclonal antibodies, vaccines, and cell therapies, revolutionize treatment outcomes.…

United States Probiotic Wellness Drinks Market to hit US$ 569.9 Million by 2031 …
Leander, Texas and Tokyo, Japan - Jan.19.2026
As per DataM intelligence research report "The Global Probiotic Wellness Drinks Market reached US$ 812.1 million in 2023 and is expected to reach US$ 1,899.9 million by 2031, growing with a CAGR of 12.9% during the forecast period 2024-2031."
The probiotic wellness drinks market is booming as consumers seek functional beverages for gut health, immunity, and overall wellness. Advanced fermentation, strain-specific probiotics, and flavor innovation…
More Releases for Viral
Viral and Non Viral Vector Manufacturing Market Share Research Report 2025
On Mar 20, 2025, Global Info Research released a research report titled "Global Viral and Non Viral Vector Manufacturing Market 2025 by Manufacturers, Regions, Type and Application, Forecast to 2031". This report provides detailed data analysis of the Viral and Non Viral Vector Manufacturing market from 2020 to 2031. Including the market size and development trends of Viral and Non Viral Vector Manufacturing Market, it analyzes market size indicators such…
Viral Inactivation Market Report 2024 - Viral Inactivation Market Size, Trends A …
"The Business Research Company recently released a comprehensive report on the Global Viral Inactivation Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The viral inactivation market size…
Viral Filtration Market - Unleashing the Power of Filtration: Safeguarding again …
Newark, New Castle, USA: The "Viral Filtration Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Viral Filtration Market: https://www.growthplusreports.com/report/viral-filtration-market/8028
This latest report researches the industry structure, sales, revenue,…
Viral Clearance Market - Unleash the Power of Clearance: Unrivaled Viral Protect …
Newark, New Castle, USA: The "Viral Clearance Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Viral Clearance Market: https://www.growthplusreports.com/report/viral-clearance-market/7823
This latest report researches the industry structure, sales, revenue,…
Viral Traffic Code - Is The Viral Traffic Code LEGIT?
Viral Traffic Code is a digital program that offers specific strategies to make affiliate earnings making use of a various technique stream. Read this review to get more information about the Viral Website Traffic Code!
Official Web Site: Go Here - https://www.glitco.com/get-Viral-Traffic-Code
What is Viral Website Traffic Code?
Viral Website Traffic Code is a basic system that might aid you make associate profits from various trusted internet sites, including Amazon, ebay.com, Shopify, ClickBank,…
Viral Inactivation Market Growing Demand of Kits and Reagents Viral Inactivation …
According to Precision Business Insights (PBI), the latest report, the Viral Inactivation market is expected to be worth USD 2.1 billion in 2022, growing at a 12.8% CAGR from 2022 to 2028. The primary driver of the expansion of the global Viral Inactivation market are speedy growth in pharmaceutical and biotechnology industries and strong inclination of R&D investments in life sciences industry.
View the detailed report description here - https://precisionbusinessinsights.com/market-reports/viral-inactivation-market/
Kits &…
