Press release
Medical-Grade TENS Replacement Pads Manufacturer Gains FDA Attention at Arab Health
Arab Health, the Middle East's premier exhibition for healthcare industry professionals, is currently in Dubai and this event has brought with it much-needed awareness about non-invasive pain management and sleep technology advancements. Careboo has distinguished itself among thousands of exhibitors showcasing medical innovations by drawing significant interest from international distributors and regulatory bodies. As a premier Medical-grade TENS replacement pads manufacturer(https://www.careboohealth.com/product/tens-unit-replacement-pads-tens-pads2%e2%80%b3x4%e2%80%b3-5x10cm-3-5mm-button-square-tens-electrodes-48pcsfive-colors-to-choose/), Careboo is using this global stage to showcase how advanced materials science and quality control can revolutionize TENS therapy efficacy. At this critical juncture in their business growth strategy, the company recently achieved regulatory milestones which will facilitate further expansion across North American and European markets.Healthcare Industry in Transition
Healthcare industries worldwide are currently experiencing an unprecedented transition, moving away from pharmaceutical dependency towards physical and technological interventions for pain management and sleep health. This transition is being spurred by two simultaneous global crises: opioid abusers needing safe alternatives for chronic pain relief; and stress and lifestyle factors leading to record rates of insomnia and sleep apnea cases.
Arab Health market analysis revealed at industry symposiums indicates that the global non-opioid pain patch and TENS market is set for exponential expansion over the coming decade. Patients and healthcare providers are seeking "wearable therapy", solutions which integrate seamlessly into daily life without side effects or unwanted side effects. Unfortunately, however, TENS units' effectiveness relies entirely on quality interface between device and human body: electrode pad. In the past, low-grade consumables often caused skin irritation or poor conductivity that undermined therapy effectiveness resulting in inadequate therapy effectiveness resulting in no effective solution being offered on market.
At the same time, sleep health services are experiencing an unprecedented convergence of diagnostics and treatments; tracking sleep alone no longer suffices - consumers increasingly demand actionable solutions that deliver real benefits. As we move toward closed-loop systems where monitoring devices connect directly with therapeutic tools for managing respiratory soft tissue or neuro-stimulation tools that facilitate relaxation, the trend toward closed-loop systems is growing increasingly popular. As populations age and the Silver Economy expands, demand for home medical devices offering professional-grade results -- from muscle recovery to joint strain relief -- has skyrocketed. Careboo's strategic position at the intersection of these trends demonstrates their deep understanding of this future trajectory where health management will increasingly occur proactively through technology rather than reactively via medications.
Careboo's Success at Arab Health Can be Attributed to Its International Certifications
Careboo has achieved incredible success at Arab Health through its impressive portfolio of international certifications, particularly when considering that medical device regulations have become increasingly stringent, particularly with European Union transition from Medical Device Directive (MDD) to more stringent Medical Device Regulation (MDR), using certification as the ultimate litmus test for quality and safety.
Careboo has successfully navigated this challenging regulatory landscape by attaining ISO13485 certification - considered the gold standard in quality management systems for medical devices - demonstrating their manufacturing processes meet international expectations of consistency and risk management. Furthermore, Careboo achieved CE Marking under MDR requirements, which verifies their products meet European Economic Area safety, health, and environmental protection regulations.
One of the highlights at Arab Health for attendees was Careboo's FDA 510(k) clearance, an important gateway into the United States market which validates that its devices and accessories are safe, effective and substantially equivalent to legally available predicate devices. When combined with RoHS compliance certification--ensuring products free from harmful substances--these certifications create a "trust moat" around Careboo.
At Arab Health, certifications are more than paperwork - they represent trust. Hospital procurement officers and pharmacy chains use these certifications as proof that Careboo is adhering to global standards, making Careboo an invaluable partner capable of meeting them all. Careboo can distribute its TENS units, heating pads, and sleep therapy monitors with complete confidence knowing their end-users can enjoy professional-grade safety in any setting imaginable.
Redefining the Interface: Medical-Grade TENS Pads and Beyond
While certifications open doors, product innovation builds loyalty. At Careboo's exhibition was an in-depth examination of their Medical-Grade TENS Replacement Pads' engineering. Careboo uses a proprietary hydrogel formulation which ensures uniform current distribution thereby eliminating hot spots--areas of intense stinging that often force users away from TENS therapy altogether. They're designed with hypoallergenic materials as well as breathable cloth backings suited for users with sensitive skin while lasting through multiple uses.
Careboo's innovative technologies extend well beyond pain management. For years now, they have been researching and developing sleep disorder technologies, and at this Expo they showcased their breakthrough progress in stopping and reducing snoring by targeting jaw muscle weakness and respiratory soft tissue softness - providing solutions that address root causes rather than mask symptoms of snoring rather than just masking symptoms. Careboo also supports its focus on "improving sleep" through advanced testing technologies which have shown highly successful results when applied clinically.
Careboo's product ecosystem exemplifies their holistic approach to wellness. They use an "multi-physics" approach, combining electrical pulses, pressure, heat, cold and light therapy. This comprehensive methodology allows them to meet a range of needs: electrical pulses; pressure; heat cold light therapy as well as light therapy can all be utilized effectively for therapeutic benefits.
Pain Relief and Muscle Strains: Utilizing TENS units and Red Light Therapy products.
Vitality & Fitness: Muscle stimulation devices that aid recovery post exercise.
Relaxation & Comfort: Utilizing ergonomic heating pads and cold compress packs designed for individual body contours can bring relaxation & comfort.
Careboo's R&D team adheres to stringent ergonomic design concepts, guaranteeing that no matter if a device is treating joint strain or monitoring sleep apnea, it fits naturally with human forms. This results in an unparalleled experience of care where high-quality materials meet cutting edge bioelectronic technology.
Careboo's presence at Arab Health marks its global ambitions and international sales of its Sleep Therapy Monitors, Snoring Stop Devices, and TENS Units are already selling worldwide. Their goal is to bring high-quality sleep enjoyment and pain-free living. Careboo sets new standards by adhering to both FDA and MDR requirements while emphasizing user experience design with each home healthcare product they make available to consumers.
As the company expands, its core mission remains the same: provide customers with superior service through high-quality products that address three fundamental pillars of health: sleep, movement and recovery.
Careboo offers medical-grade solutions, certifications, and products suited for hospital environments. For more information about them, visit their official website.
Media Contact for Careboo Health can be found here: CarebooHealth.com/
Telephone
+86-13760204436
Mailbox
qd0755@163.com
Address
No. 7 Chuangye Road Dongshan Village, Qishi Town, Dongguan City
Careboo focuses on sleep health and healthcare related products. Careboo has long been committed to the research, development, and technology of various sleep disorders and pain disorders.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Medical-Grade TENS Replacement Pads Manufacturer Gains FDA Attention at Arab Health here
News-ID: 4345759 • Views: …
More Releases from CAREBOO CO., LIMITED

China CAREBOO Respiratory Device Manufactor CE/FDA Highlights Compliance Strengt …
Careboo, a China CE/FDA respiratory device manufacturer(https://www.careboohealth.com/product-category/snoring/), is delighted to join Arab Health 2026 at Dubai Exhibition Centre as an exhibitor and take advantage of this premier platform to demonstrate their latest advancements in sleep health and respiratory therapy, emphasizing their rigorous commitment to international safety standards and therapeutic efficacy for an international audience.
The Global Sleep Health Horizon: Industry Prospects and Trends
The global market for sleep apnea and respiratory devices…
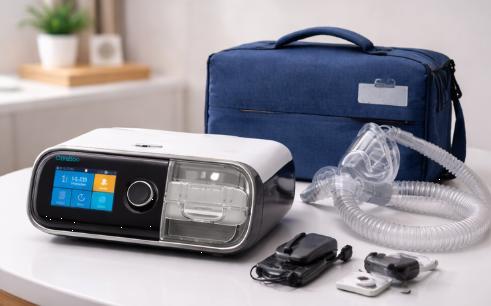
CAREBOO Air Breathing Equipment Manufacturer China Introduces New Respiratory Sy …
As global respiratory awareness and the prevalence of sleep-related breathing disorders increase, so has the demand for advanced, safe, and patient-centric respiratory systems. CAREBOO air breathing equipment manufacturer China is emerging as a leader in this space-offering high-quality solutions across hospitals, sleep clinics, and homecare settings. Its innovative range combines advanced technology, rigorous regulatory compliance, and patient-centric design, setting benchmarks in respiratory therapy while unveiling its latest innovations at the…

MDR Compliant Breathing Device Manufactor China vs Low-Cost Alternatives
Global healthcare standards have reached a crucial turning point, prompting an unprecedented need for high-performance respiratory therapy solutions. At Careboo, a pioneering sleep health and advanced medical technology company, care is taken in serving as a premier MDR compliant breathing device manufactor China(https://www.careboohealth.com/product-category/snoring/) while prioritizing clinical safety. By adhering to the rigorous EU 2017/745 Medical Device Regulation and prioritizing clinical safety and regulatory alignment over standard sleep aids, Careboo provides…

How CE Approved Auto Ventilator Wholesale China Meets Rising Clinical Requiremen …
CE approved auto ventilator wholesale China(https://www.careboohealth.com/product-category/snoring/) has become an indispensable sourcing channel for hospitals, distributors, and healthcare providers looking for high-quality devices that balance safety with performance and scalability. Leading this transformation is Careboo, an healthcare technology company dedicated to improving sleep health through innovation, compliance, and patient-centric design.
Emergence of Clinical Demand for Automatic Ventilators
Since 2008, clinical requirements for automatic ventilators have steadily grown over time. Occlusive Sleep Apnea (OSA),…
More Releases for TENS
Guide to Working with Reusable TENS Electrodes Manufactory CAREBOO
Brand owners are looking for manufacturing partners who can offer more than just assembly lines. They want a combination of clinical expertise, innovation and regulatory reliability. Careboo has become a leader in the therapeutic technology industry and set new standards for international partnerships. By collaborating with the Reusable TENS electrodes manufacturer CAREBOO(https://www.careboohealth.com/product-category/electric-therapy/tens-units/), entrepreneurs and established medical devices companies can expand their portfolios by offering eco-friendly, high-durability solutions. Careboo provides a…
Rising Consumer Health Trends Boost CAREBOO EMS & TENS Therapy Device Manufactor …
The global health consciousness is increasing, and the demand for home-based medical devices that are sophisticated has reached new heights. Careboo is a leading innovator in the wellness sector and strategically positioned to satisfy this demand. CAREBOO TENS & EMS therapy device manufacturer(https://www.careboohealth.com/product-category/electric-therapy/) is a hub that produces high-quality medical devices by aligning advanced research and the growing trend toward self-care. Careboo's commitment to integrating digital precision with physical therapy…
Volkswagen plans to cut tens of thousands of employees
Image: https://www.chinashinyfly.com/uploads/W.jpg
Management plans to close at least three local factories and cut tens of thousands of employees to cut operating costs, he said at a staff event at Volkswagen [https://www.chinashinyfly.com/b24f-plastic-quick-connector-for-fuel-system-%cf%867-89-516%e3%80%9e-id6-90-sae-product/] headquarters in Wolfsburg on October 28.
Cavallo said the board had carefully considered the plan and that all German factories could be affected by the closure plan and that other workers who had not been closed would also face pay cuts.…
TENS Machine Market Size 2024 to 2031.
Market Overview and Report Coverage
A Transcutaneous Electrical Nerve Stimulation (TENS) Machine is a device used to provide pain relief through electrical stimulation. The TENS Machine Market is expected to grow at a CAGR of 6.80% during the forecasted period.
The future outlook of the TENS Machine Market is promising due to the increasing prevalence of chronic pain conditions, rising aging population, and growing awareness about non-invasive pain management…
Global TENS Machine Market Research Report
This report studies the global TENS Machine market status and forecast, categorizes the global TENS Machine market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in North America, Europe, Japan, China, and other regions (India, Southeast Asia). The major manufacturers covered in this report HealthmateForever Zewa Omron TruMedic TechCare PurePulse IReliev Therapeutix Geographically, this report studies the top producers and consumers,…
PKB registers tens of thousands of patients with mass registration
Patients Know Best (PKB) - a social enterprise providing the world’s first patient-controlled health records, has introduced ‘mass registration’ enabling patients to sign up to access their health records at scale and with speed.
Following a number of successful trials in partnership with NHS organisations, patients can now sign up for their health record in a number of ways; either by speaking to a member of staff; by using the…
