Press release
In-Vitro Diagnostics (IVD) Market to Reach US$ 131.68 Billion by 2032 at 5.0% CAGR; North America Leads with 36% Share - Key Players: Roche, Abbott, Siemens Healthineers
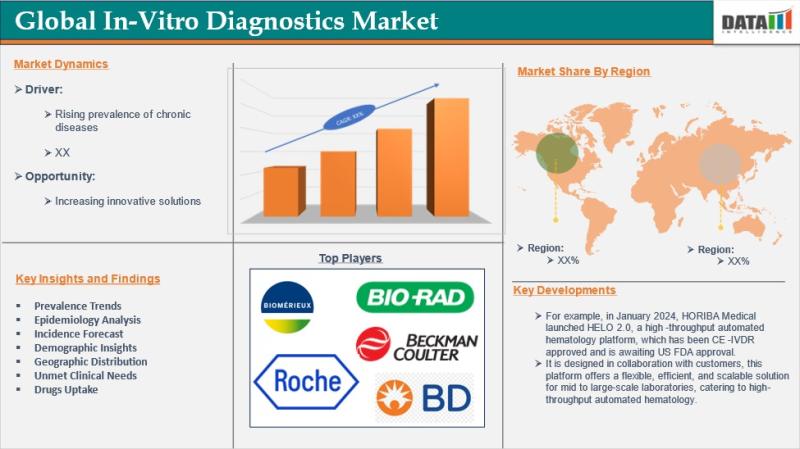
The global In-Vitro Diagnostics (IVD) Market reached US$ 84.90 billion in 2024 and is expected to reach US$ 131.68 billion by 2032, growing at a CAGR of 5.0% during the forecast period 2025-2032. Market growth is driven by the rising prevalence of chronic and infectious diseases, increasing demand for early and accurate diagnosis, and continuous advancements in diagnostic technologies.In-vitro diagnostics are medical tests performed outside the human body to detect diseases, conditions, and infections, typically using samples such as blood, urine, or tissue. These tests are conducted across diverse settings, including centralized laboratories, hospitals, clinics, and home-care environments, utilizing instruments ranging from portable point-of-care devices to advanced automated laboratory systems. IVDs play a critical role in clinical decision-making by enabling accurate diagnosis, disease monitoring, and personalized treatment planning. Growing emphasis on preventive healthcare, expansion of home-based testing, and technological innovations in molecular diagnostics and immunoassays are further supporting the sustained growth of the global in-vitro diagnostics market.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://www.datamintelligence.com/download-sample/in-vitro-diagnostics-market?sai-v
The In-Vitro Diagnostics (IVD) Market refers to the global industry focused on medical tests and devices used to detect diseases, conditions, or infections by analyzing samples such as blood or tissue outside the human body.
Key Developments
✅ January 2026: IVD manufacturers increased focus on AI-enabled diagnostic platforms, integrating machine learning into clinical decision support to improve accuracy in oncology, cardiology, and infectious disease testing.
✅ December 2025: Demand for point-of-care and near-patient testing solutions rose sharply across hospitals and diagnostic labs, driven by faster turnaround time requirements and decentralized healthcare delivery models.
✅ November 2025: Regulatory authorities approved multiple high-throughput molecular diagnostic assays, strengthening testing capabilities for respiratory infections and antimicrobial resistance detection.
✅ October 2025: Clinical laboratories accelerated adoption of automated sample preparation and digital pathology systems to improve workflow efficiency and reduce manual errors.
✅ September 2025: Increased investment was observed in companion diagnostics linked to targeted therapies, particularly in oncology and rare disease management.
✅ August 2025: IVD companies expanded manufacturing capacity for reagents and consumables to address supply stability concerns and support growing global testing volumes.
Mergers & Acquisitions
✅ December 2025: A leading multinational diagnostics company acquired a specialty molecular diagnostics firm to expand its oncology and genetic testing portfolio.
✅ October 2025: A major life sciences company completed the acquisition of an automation and laboratory software provider to strengthen end-to-end IVD workflow solutions.
✅ September 2025: Strategic acquisition activity increased among mid-size IVD players, focusing on point-of-care technologies and rapid diagnostic platforms to enhance competitive positioning.
Key Players
bioMérieux | Becton, Dickinson and Company | Beckman Coulter, Inc. | F. Hoffmann-La Roche Ltd. | Bio-Rad Laboratories, Inc. | Abbott Laboratories | Qiagen NV | FUJIFILM Holdings Corporation | Sysmex Corporation | Siemens Healthineers AG
Key Highlights
F. Hoffmann-La Roche Ltd. - Holds 19.6% share, driven by strong leadership in molecular diagnostics, immunoassays, and companion diagnostics, supported by continuous innovation and global clinical adoption.
Abbott Laboratories - Holds 17.8% share, supported by a broad IVD portfolio spanning immunoassays, clinical chemistry, and point-of-care diagnostics, along with strong global distribution.
Siemens Healthineers AG - Holds 15.2% share, leveraging advanced laboratory automation, high-throughput analyzers, and strong presence across hospital and reference laboratories.
Becton, Dickinson and Company - Holds 12.9% share, driven by strength in microbiology, flow cytometry, and diagnostic systems widely adopted in clinical laboratories.
Sysmex Corporation - Holds 10.4% share, supported by leadership in hematology diagnostics, automation solutions, and expanding adoption in emerging markets.
bioMérieux - Holds 8.7% share, benefiting from strong positioning in infectious disease diagnostics and microbiology testing solutions.
Beckman Coulter, Inc. - Holds 7.6% share, driven by clinical chemistry and immunoassay systems, with strong integration in high-volume laboratory settings.
Qiagen NV - Holds 4.9% share, supported by molecular diagnostics expertise, sample preparation technologies, and growing demand for precision medicine.
Bio-Rad Laboratories, Inc. - Holds 4.1% share, focused on specialty diagnostics, quality controls, and life science research applications.
FUJIFILM Holdings Corporation - Holds 3.8% share, leveraging imaging-based diagnostics, digital pathology, and expanding IVD product offerings.
Purchase this report before year-end and unlock an exclusive 30% discount: https://www.datamintelligence.com/buy-now-page?report=in-vitro-diagnostics-market?sai-v
(Purchase 2 or more Reports and get 50% Discount)
Market Drivers
- Rising prevalence of chronic and infectious diseases increasing the need for early, accurate, and large-scale diagnostic testing.
- Growing global aging population leading to higher demand for routine diagnostics, disease monitoring, and preventive screening.
- Increasing adoption of personalized medicine and companion diagnostics, especially in oncology and rare disease treatment.
- Technological advancements in molecular diagnostics, immunoassays, and point-of-care testing improving diagnostic accuracy and speed.
- Expansion of healthcare infrastructure, diagnostic laboratories, and testing facilities in emerging economies supporting higher test volumes.
Industry Developments
- Continuous innovation in molecular diagnostics technologies such as PCR, next-generation sequencing, and multiplex testing platforms.
- Rising adoption of automated and high-throughput diagnostic systems to improve laboratory efficiency and reduce turnaround times.
- Development and commercialization of rapid and point-of-care IVD tests enabling decentralized and near-patient diagnostics.
- Strategic partnerships, acquisitions, and collaborations among IVD companies to expand product portfolios and geographic reach.
- Increased integration of digital diagnostics, data analytics, and AI-driven tools to support clinical decision-making and workflow optimization.
Regional Insights
North America - 36% share: "Driven by advanced healthcare infrastructure, strong reimbursement systems, high adoption of innovative diagnostic technologies, and significant demand for molecular and companion diagnostics."
Europe - 28% share: "Supported by established diagnostic laboratory networks, government-backed screening programs, and growing emphasis on preventive healthcare and early disease detection."
Asia Pacific - 30% share: "Fueled by expanding healthcare access, rising disease burden, increasing investments in laboratory infrastructure, and rapid adoption of cost-effective diagnostic solutions across developing economies."
Latin America - 4% share: "Driven by improving healthcare systems, expanding private diagnostic laboratory networks, and increasing awareness of early disease diagnosis."
Middle East & Africa - 2% share: "Supported by healthcare infrastructure development, rising investments in diagnostic services, and growing focus on infectious disease testing and public health programs."
Speak to Our Analyst and Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/in-vitro-diagnostics-market?sai-v
Key Segments
By Product Type
Instruments represent a major share of the market, driven by continuous demand for advanced diagnostic platforms and laboratory automation. Reagents hold a significant portion, supported by recurring consumption and increasing test volumes across diagnostic settings. Software and services are witnessing strong growth, driven by the need for data management, workflow optimization, and integrated diagnostic solutions. Other product types contribute steadily through specialized and niche diagnostic offerings.
By Technique Type
Immunoassays dominate the market due to their wide use in disease screening, monitoring, and routine diagnostics. Point-of-care diagnostics are experiencing rapid growth, supported by demand for rapid testing and decentralized healthcare delivery. Molecular diagnostics hold a substantial share, driven by high sensitivity, specificity, and growing adoption in infectious disease and genetic testing. Hematology remains a core segment due to routine blood analysis requirements. Tissue diagnostics and microbiology contribute significantly through pathology and infectious disease testing, while self-blood-glucose monitoring continues to expand with rising diabetes prevalence. Other techniques support specialized diagnostic applications.
By Application
Infectious diseases represent the leading application segment, driven by rising disease burden and increased diagnostic testing. Diabetes holds a significant share, supported by long-term monitoring needs and high prevalence rates. Oncology is expanding steadily due to growing cancer incidence and advancements in precision diagnostics. Cardiology applications are supported by increasing cardiovascular disorders and preventive screening. Autoimmune diseases and nephrology contribute notably through specialized testing, while drug testing and other applications support broader diagnostic demand.
By End-User
Diagnostic laboratories dominate the end-user segment, driven by high testing volumes and centralized diagnostic services. Hospitals hold a substantial share, supported by integrated diagnostic facilities and inpatient testing requirements. Academic and research institutes contribute steadily through clinical research and diagnostic innovation, while other end-users, including specialty clinics and home-care settings, support overall market growth.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In-Vitro Diagnostics (IVD) Market to Reach US$ 131.68 Billion by 2032 at 5.0% CAGR; North America Leads with 36% Share - Key Players: Roche, Abbott, Siemens Healthineers here
News-ID: 4336829 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Japan Pharmaceuticals Market (2025-2032) | Market to Reach USD 206.70 billion by …
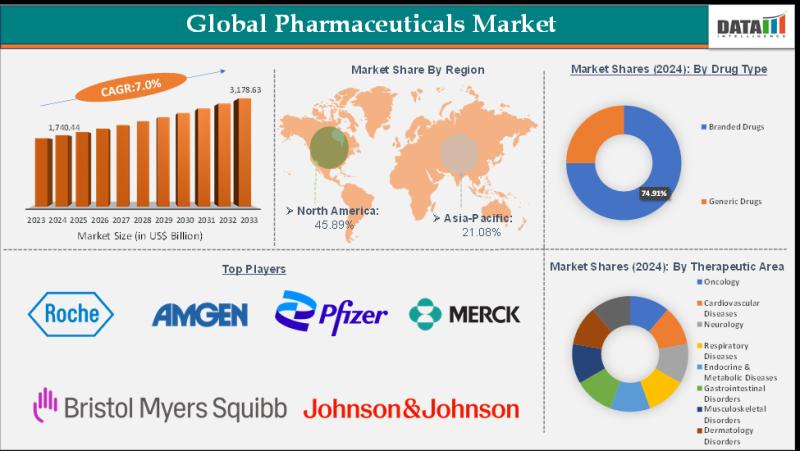
The Japan Pharmaceutical Market was valued at approximately USD 71.51 billion in 2024 and is projected to grow to USD 103.05 billion by 2030 at a CAGR of 6.3%, with some estimates forecasting it to reach USD 206.70 billion by 2032 during the Forecast period 2025-2032
Global Pharmaceutical market size grew from US$ 1,635.82 billion in 2023 to US$ 1,740.44 billion in 2024 and is projected to reach US$ 3,178.63 billion…

Global Aluminum Gallium Arsenide Market Set for Explosive Growth to USD 4.2 Bill …
The Global Aluminum Gallium Arsenide Market reached USD 1.9 billion in 2023 and is expected to reach USD 4.2 billion by 2031, growing with a CAGR of 10.2% during the forecast period 2024-2031.
Market growth is driven by surging demand in high-speed semiconductors, optoelectronics, and 5G/6G infrastructure, alongside expanding applications in solar cells, LEDs, and laser diodes. Advancements in epitaxial growth techniques, rising investments in photonics and RF devices, growing adoption…

Smart Office Market to Reach US$ 122.36 Billion by 2032 at 12.8% CAGR | IoT Enab …
Smart Office Market reached US$ 48.22 billion in 2024 and is expected to reach US$ 122.36 billion by 2032, growing at a CAGR of 12.8% during the forecast period 2025 to 2032.
Market growth is driven by rapid workplace digital transformation, increasing adoption of IoT enabled building management systems, and rising demand for energy efficient, secure, and highly connected office environments. Organizations are investing in smart lighting, intelligent HVAC controls, occupancy…
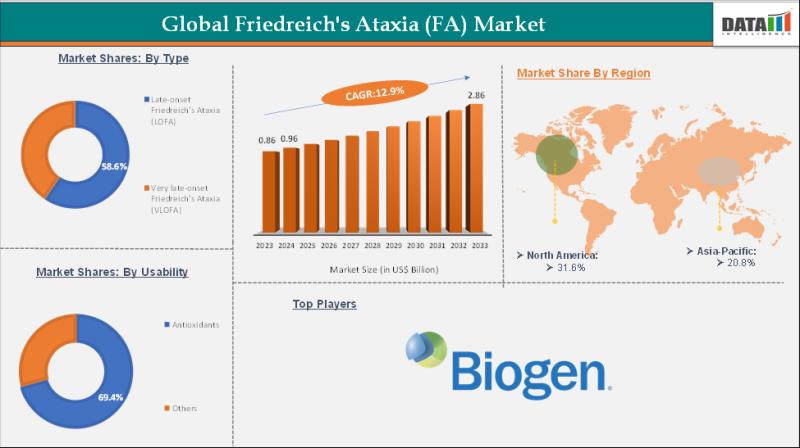
United States Friedreich's Ataxia (FA) Market Growth (2025-2033): Emerging Thera …
DataM Intelligence has published a new research report on "Friedreich's Ataxia Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.
✅ North America is projected to maintain a…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…