Press release
United States Neuromuscular Disease Therapeutics Market Outlook 2025-2033 | CAGR 14.4% | North America Leads with 45% | Key Players: Novartis, Biogen, Roche, Sarepta, AbbVie
Neuromuscular Disease Therapeutics MarketThe neuromuscular disease therapeutics market reached US$ 11.89 billion in 2023, rising to US$ 13.70 billion in 2024, and is projected to reach US$ 45.62 billion by 2033, growing at a CAGR of 14.4% during the forecast period 2025-2033.
The market is expanding as advances in genetic medicine and precision therapies are redefining treatment possibilities for rare, debilitating neuromuscular disorders. Spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD) currently dominate the market, driven by high-impact approvals such as Spinraza, Zolgensma, Evrysdi, and Elevidys, which have demonstrated both clinical efficacy and commercial success. Increasing adoption of newborn screening programs, particularly for SMA, is identifying patients at a presymptomatic stage, where early intervention can deliver the greatest therapeutic benefit.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/neuromuscular-disease-therapeutics-market?Juli
Recent Developments:
✅ Nov 2025: The FDA approved Novartis' Itvisma (onasemnogene abeparvovec‐brve), an intrathecal gene‐replacement therapy for people with spinal muscular atrophy (SMA) aged 2 years and older, expanding access to gene therapy beyond infancy.
✅ Jun 2025: The European Commission approved Roche's Evrysdi tablet formulation as the first and only non‐invasive treatment for SMA, offering improved convenience, room‐temperature stability, and simplified dosing.
✅ Feb 2025: The U.S. FDA granted approval for Evrysdi in tablet form for SMA, providing patients with an easy‐to‐administer option alongside the existing oral solution.
✅ Jun 2024: Long‐term data from Roche's Evrysdi (risdiplam) showed sustained benefits in children with Type 1 SMA, with high survival and functional outcomes after five years of treatment.
✅ Jun 2024: The U.S. FDA expanded approval of Elevidys (delandistrogene moxeparvovec‐rokl) for Duchenne muscular dystrophy (DMD) in patients aged 4 and older, covering both ambulatory and non‐ambulatory individuals with a confirmed DMD mutation.
✅ Sep 2025: Dyne Therapeutics received Orphan Drug designation in Japan for DYNE‐251 in DMD, based on sustained functional improvement in the ongoing Phase 1/2 DELIVER trial.
Mergers & Acquisitions:
✅ July 2024: Biogen acquired a minority stake in Beam Therapeutics, strengthening its pipeline in gene-editing therapies for neuromuscular diseases.
✅ May 2024: Roche entered a strategic collaboration with Audentes Therapeutics, expanding its portfolio in AAV-based gene therapies for DMD and SMA.
✅ March 2024: Sarepta Therapeutics partnered with Sana Biotechnology to co-develop advanced gene therapy modalities, focusing on enhanced delivery and long-term efficacy for muscular dystrophies.
✅ January 2024: Pfizer completed acquisition of Krystal Biotech's neuromuscular division, enhancing its capabilities in rare disease therapeutics and expanding its SMA and DMD pipeline.
✅ November 2023: AveXis (a Novartis company) announced a strategic collaboration with CureDuchenne, aiming to accelerate clinical research and patient access programs for Duchenne muscular dystrophy therapies.
Buy Now & Unlock 360° Market Intelligence:-https://www.datamintelligence.com/buy-now-page?report=neuromuscular-disease-therapeutics-market?Juli
Key Players:
• Novartis - Leader in SMA gene therapy with Itvisma (onasemnogene abeparvovec‐brve) and Spinraza collaborations.
• AbbVie - Focused on innovative therapies for neuromuscular disorders, including pipeline candidates in DMD and ALS.
• Biogen - Developer of Spinraza for SMA, with ongoing research in antisense oligonucleotide therapies and combination treatments.
• AstraZeneca - Active in rare neuromuscular disease research and early-stage gene therapy collaborations.
• Argenx - Specializes in antibody-based therapies targeting neuromuscular autoimmune disorders such as myasthenia gravis.
• Sarepta Therapeutics - Leader in Duchenne muscular dystrophy therapies, including Elevidys and next-generation gene therapies.
• Takeda - Focused on rare neuromuscular disorders through gene therapy and antisense approaches, with an emphasis on global expansion.
• Nippon Shinyaku - Developing therapies for SMA and other neuromuscular conditions, with a strong presence in the Japanese market.
• Grifols - Active in plasma-derived treatments and therapies targeting autoimmune-mediated neuromuscular disorders.
Market Segmentation:
➥By therapy, the neuromuscular disease therapeutics market is led by gene therapies, accounting for an estimated 42% of total market value as of 2025, driven by high‐impact approvals and strong uptake in spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD). Antisense oligonucleotides (ASOs) represent approximately 25%, underpinned by established products such as Spinraza and emerging next‐generation candidates. Small‐molecule splicing modulators hold about 14%, benefiting from oral convenience and expanding indications. Monoclonal antibodies and complement inhibitors contribute roughly 11%, with therapies targeting autoimmune neuromuscular disorders such as myasthenia gravis. The others category, including enzyme replacement and novel modalities such as mRNA‐based therapeutics, constitutes the remaining 13% of the market.
➥From a disease type perspective, spinal muscular atrophy (SMA) captures the largest share at around 29%, followed by muscular dystrophy (primarily DMD) at 27%. Amyotrophic lateral sclerosis (ALS) and myasthenia gravis each account for approximately 10%, driven by growing clinical pipelines. Peripheral neuropathy and myositis together represent about 14%, while Charcot‐Marie‐Tooth disease, multiple sclerosis (MS), myopathy, and others collectively make up the balance of the market (17%).
➥By route of administration, intravenous (IV) therapies dominate with approximately 45% share, reflecting their use in gene therapies, ASOs, and monoclonal antibodies. Oral routes are capturing an increasing share at 31%, largely due to small‐molecule splicing modulators and tablet formulations gaining regulatory approvals. Intrathecal administration represents roughly 17%, primarily for gene therapies delivered directly to the central nervous system. Subcutaneous (SC) delivery accounts for about 9%, and other emerging routes (e.g., intramuscular) comprise the remaining 4% of market value.
➥In terms of distribution channels, hospital pharmacies remain the largest channel with about 46% share, reflecting their role in administering high‐cost biologics and gene therapies. Specialty clinics and centers follow with 31%, especially for advanced neuromuscular care and infusion‐based treatments. Retail pharmacies account for roughly 20%, driven by self‐administered oral and injectable therapies, while online pharmacies hold an emerging 7% share as regulatory environments evolve to support direct‐to‐patient distribution.
Speak to Our Analyst and Get Customization in the report as per your requirements:-https://www.datamintelligence.com/customize/neuromuscular-disease-therapeutics-market?Juli
Market Dynamics:
Driver: Breakthrough Approvals Validating New Modalities
Breakthrough approvals are transforming the neuromuscular disease therapeutics market by demonstrating that advanced modalities can deliver truly disease‐modifying outcomes. Therapies such as Spinraza, Zolgensma, Evrysdi, and Elevidys have shifted the treatment paradigm from symptomatic management to interventions that meaningfully alter disease progression in conditions like spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD). These landmark approvals not only expand treatment options for patients but also foster confidence among regulators, payers, and clinicians, creating a more favorable environment for pipeline therapies. By validating new scientific approaches and attracting sustained investment, they are accelerating innovation and positioning neuromuscular therapeutics as a high-growth segment within rare disease and genetic medicine.
Restraint: Manufacturing & Scalability Challenges
Despite strong innovation, manufacturing and scalability remain significant restraints in the neuromuscular disease therapeutics market. Advanced therapies, including gene therapies, antisense oligonucleotides (ASOs), and other complex biologics, require highly specialized manufacturing processes, state-of-the-art facilities, and stringent quality control. These factors increase production costs, limit supply capacity, and often result in long lead times and access challenges. Consequently, supply shortages and pricing pressures can slow adoption, particularly in emerging markets, posing hurdles to the broad, global implementation of these transformative therapies.
Regional Insights:
North America dominates the neuromuscular disease therapeutics market, accounting for approximately 45% of the global share in 2025. Growth in the region is driven by the rapid adoption of advanced therapies, robust R&D infrastructure, and strong reimbursement support. The U.S. leads the market with approvals of therapies such as Spinraza, Zolgensma, and Evrysdi, while expanding newborn screening programs for spinal muscular atrophy (SMA) are enabling early diagnosis and treatment. Canada also contributes, benefiting from public-private partnerships and increasing access to gene and antisense therapies.
Europe holds the second-largest market share at around 28%, supported by well-established regulatory frameworks and reimbursement systems. Key markets such as Germany, France, and the U.K. are seeing strong adoption of gene therapies and antisense oligonucleotides, bolstered by orphan drug incentives and early access programs. Collaborative efforts between biotech companies and academic institutions further accelerate clinical development for rare neuromuscular disorders in the region.
Asia Pacific represents approximately 20% of the global market and is an emerging high-growth region due to rising awareness, expanding healthcare infrastructure, and regulatory support for orphan drugs. Japan is the leading market in the region, with companies like Nippon Shinyaku and Takeda actively developing SMA and Duchenne muscular dystrophy (DMD) therapies. China, South Korea, and India are witnessing gradual adoption of gene therapies and increased clinical trial activity, offering significant growth potential over the forecast period.
📌 Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
☛ Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
☛ Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg?Juli
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States Neuromuscular Disease Therapeutics Market Outlook 2025-2033 | CAGR 14.4% | North America Leads with 45% | Key Players: Novartis, Biogen, Roche, Sarepta, AbbVie here
News-ID: 4334897 • Views: …
More Releases from DataM intelligence 4 Market Research LLP

Digital Oilfield Market to Reach 7.1% CAGR by 2032; North America Leads with 40% …
Market Overview
The Global Digital Oilfield Market is witnessing significant growth, with a CAGR of 7.1% during the forecast period. The market is driven by increasing adoption of advanced software and data analytics in oil and gas operations, enabling operators to enhance field productivity, reduce operational costs, and optimize decision-making processes.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/global-digital-oilfield-market?Juli
A digital oilfield leverages technology to simulate…

Hydropower Market to Reach US$ 348.2 Billion by 2030; Asia-Pacific Leads with 45 …
Market Overview
The global hydropower market reached US$ 244.1 billion in 2022 and is projected to reach US$ 348.2 billion by 2030, growing at a CAGR of 4.8% during 2024-2031. Hydropower continues to be a key renewable energy source worldwide, driven by innovations in turbine technology, dam design, and grid integration solutions. Although hydropower has a history of nearly 150 years, recent developments focus on improving efficiency, environmental sustainability, and integration…
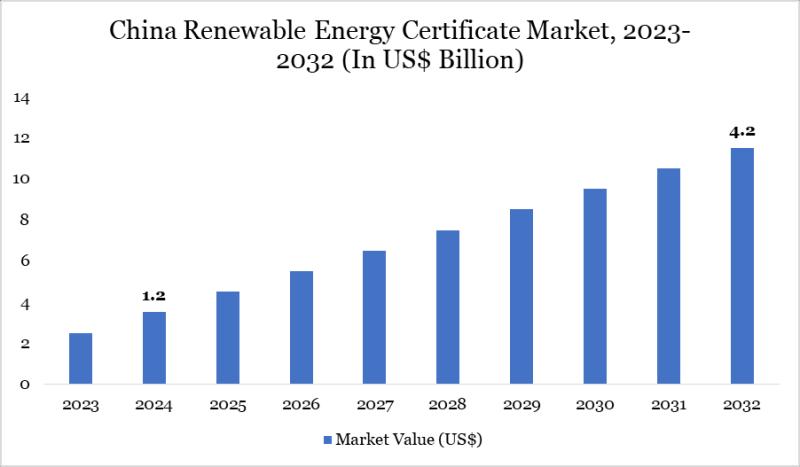
China Renewable Energy Certificate Market to Reach US$ 4.2 Billion by 2032; Asia …
Market Overview
The China renewable energy certificate (REC) market reached US$ 1.2 billion in 2024 and is expected to reach US$ 4.2 billion by 2032, growing at a CAGR of 14.2% during the forecast period 2025-2032. China has been the world's largest and fastest-growing producer of renewable energy for over a decade, generating more than three times the renewable electricity of the United States. Its market share in RECs is significant…
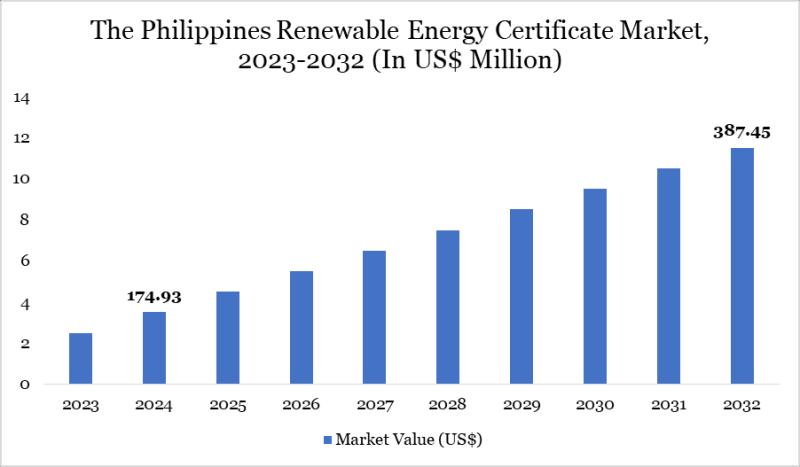
Philippines Renewable Energy Certificate Market to Reach US$ 387.45 Million by 2 …
Market Overview
The Philippines Renewable Energy Certificate (REC) Market reached US$ 174.93 million in 2024 and is projected to reach US$ 387.45 million by 2032, growing at a CAGR of 9.8% during the forecast period 2025-2032. The growth is driven by the country's strong commitment to renewable energy development, supported by government policies and incentives promoting clean energy adoption. The Philippines possesses abundant renewable resources, including solar, wind, hydro, and geothermal…
More Releases for SMA
SMA Estimating Helps Builders Bid Smarter with Accurate Cost Data
Image: https://www.abnewswire.com/upload/2026/02/acbcac80c248c2f1125434210702200c.jpg
Winning construction bids isn't just about offering the lowest price-it's about submitting a bid that's realistic, profitable, and backed by solid data. Many builders, especially small to mid-sized contractors, struggle with inaccurate numbers, rushed takeoffs, or outdated pricing. That's where SMA Estimating [https://www.smaestimating.com/] steps in to make the bidding process smarter and more reliable.
At its core, accurate cost estimating is about clarity. Builders need to know exactly how much…
Global SMA Connector Market Imapct of AI and Automation
SMA Connector Market Impact of AI and Automation
The SMA connector market experienced a notable growth trajectory in 2022, with the market size reaching approximately USD 1.2 billion. This expansion reflects a compound annual growth rate (CAGR) of 6.3% from the previous year. The increasing demand for high-frequency communication and advancements in telecommunications infrastructure have driven this growth. SMA connectors are integral in various applications, including radio frequency (RF) systems, and…
RF SMA Connector Market Analysis and Future Prospects for 2030
The rf sma connector market represents a multifaceted and continually evolving realm, influenced by shifting consumer demands and technological advancements. In this comprehensive report, we embark on a thorough exploration of this market landscape, catering to a diverse audience ranging from manufacturers and suppliers to distributors and investors. Our primary objective is to arm industry stakeholders with indispensable insights, enabling them to make well-informed decisions within the swiftly changing market…
Battery Storage Market is Booming Worldwide | Dynapower, SMA, KACO
Advance Market Analytics published a new research publication on "Battery Storage Market Insights, to 2030" with 232 pages and enriched with self-explained Tables and charts in presentable format. In the Study you will find new evolving Trends, Drivers, Restraints, Opportunities generated by targeting market associated stakeholders. The growth of the Battery Storage market was mainly driven by the increasing R&D spending across the world.
Get Free Exclusive PDF Sample Copy of…
Global SMA Attenuator Market Research Report 2023-2029
Global SMA Attenuator Market: Driven factors and Restrictions factors
The research report encompasses a comprehensive analysis of the factors that affect the growth of the market. It includes an evaluation of trends, restraints, and drivers that influence the market positively or negatively. The report also outlines the potential impact of different segments and applications on the market in the future. The information presented is based on historical milestones and current trends,…
Spinal Muscular Atrophy Market | SMA Treatment Market | PBIGP
Spinal Muscular Atrophy Management Market is Expected to Increase Around US$ XX Mn by 2025, Due to Inherited Diseases that Affects the Functioning of Muscles Because of Deterioration.
Analyst Speak:
“The Spinal Muscular Atrophy Management Market is anticipated to reach about US$ XX Mn by 2024, and anticipated to expand at a CAGR over seven years of forecast period 2019-2025” due to increased inherited diseases that affects the normal functioning of…
