Press release
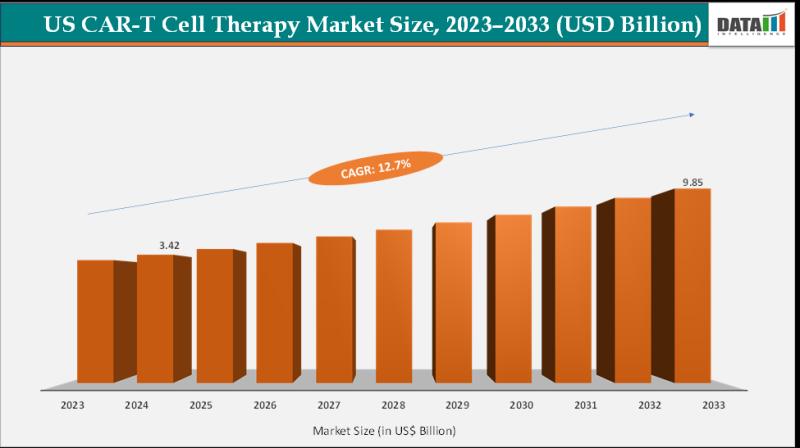
United States CAR-T Cell Therapy Market set for steady growth to US$ 9.85 billion by 2033 at a CAGR of 12.7% | Key Players:- Novartis, Gilead/Kite Pharma, Bristol Myers Squibb
The U.S CAR-T cell therapy market size reached US$ 3.42 billion in 2024 from US$ 3.07 billion in 2023 and is expected to reach US$ 9.85 billion by 2033, growing at a CAGR of 12.7% during the forecast period 2025-2033.This growing number of FDA-approved CAR-T cell therapies in the US, targeting key antigens like CD19 and BCMA, is a major driver of market expansion. These approvals validate the clinical efficacy of CAR-T treatments and broaden their use across multiple hematologic cancers such as ALL, LBCL, CLL, and multiple myeloma.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):-https://www.datamintelligence.com/download-sample/us-car-t-cell-therapy-market?pratik
United States: Key Industry Developments
✅ October 2025: Kincell Bio partnered with Moonlight Bio to advance Moonlight's lead T-cell therapy program into clinical trials, focusing on innovative CAR-T designs for enhanced efficacy in hematologic cancers.
✅ September 2025: Made Scientific and Hemogenyx Pharmaceuticals formed a manufacturing partnership to scale HG-CT-1 CAR-T therapy, targeting improved production for relapsed/refractory blood cancers amid rising U.S. demand.
✅ May 2025: Researchers demonstrated "armored" CAR-T therapy huCART19-IL18 achieving 52% complete remission in pre-treated lymphoma patients, boosting optimism for next-generation applications in non-Hodgkin lymphoma.
Key Merges and Acquisitions(2025):
Bristol Myers Squibb (Orbital Therapeutics) - October 15, 2025
Bristol Myers Squibb solidified its leadership in the US CAR-T cell therapy market by acquiring Orbital Therapeutics for $1.5 billion, focusing on the investigational in vivo CAR-T therapy OTX-20 (also referenced as OTX-201). This deal enhances BMS's cell therapy portfolio with RNA-based technologies that reprogram immune cells directly in the body, reducing manufacturing complexities and targeting autoimmune diseases alongside oncology indications.
Gilead Sciences' Kite Pharma (Interius BioTherapeutics) - August 21, 2025
Gilead's Kite Pharma strengthened its CAR-T dominance in the US through the $350 million acquisition of Philadelphia-based Interius BioTherapeutics, integrating an innovative in vivo CAR-T platform that generates CAR-T cells via a single IV infusion. The move advances Kite's pipeline beyond ex vivo therapies like Yescarta, aiming to simplify treatments, cut costs, and broaden access for blood cancer patients.
Market Segmentation Analysis:
-By Target Antigen: CD19 vs BCMA vs Others
CD19 dominates with 61.87% market share in 2024, driven by its early FDA approvals for B-cell malignancies like ALL and lymphomas, including therapies such as Kymriah and Yescarta.
BCMA holds around 25% share (based on revenue proportions), targeting multiple myeloma with products like Abecma and Carvykti, and is poised for fastest growth at 24.17% CAGR.
Others (CD22, GD2, etc.) account for the remainder, with emerging applications in solid tumors and additional blood cancers.
-By Type of Therapy: Autologous vs Allogeneic
Autologous CAR-T therapies lead with 81.1% market share in 2024, favored for personalized use of patient cells reducing rejection risks in hematologic cancers.
Allogeneic variants hold the remaining 19%, gaining traction for off-the-shelf scalability and faster access, with 23.21% CAGR projected through 2034.
-By Indication: Hematologic Malignancies vs Solid Tumors
Hematologic malignancies capture 93.85% share in 2024, led by lymphoma (largest sub-segment) and leukemia, due to proven efficacy in relapsed/refractory cases.
Solid tumors comprise the rest at 6%, with rapid growth at 23.66% CAGR amid R&D to overcome tumor microenvironment challenges.
-By End-User: Hospitals Lead with 44.0%
Hospitals command 44.0% share in 2024 (revenue $1,993 million), as primary sites for complex administration and side-effect management like CRS.
Cancer treatment centers follow at 29% ($1,347 million), with fastest growth via specialized adoption; others include academic institutes (16%), clinics (9%), and CDMOs (9%).
Purchase this report before year-end and unlock an exclusive 30% discount:https://www.datamintelligence.com/buy-now-page?report=us-car-t-cell-therapy-market?pratik (Purchase 2 or more Reports and get 50% Discount)
Growth Drivers:
Rising cancer prevalence, especially hematological malignancies such as leukemia, lymphoma, and multiple myeloma.
Strong FDA approvals and regulatory support accelerating commercialization of new CAR-T therapies.
Growing adoption of personalized & precision medicine, driving demand for targeted immunotherapies.
Advancements in CAR-T technology including next-generation allogeneic ("off-the-shelf"), dual-targeted, and safer constructs.
Expanding clinical pipeline with significant investments from pharma & biotech companies in R&D.
Increasing availability of treatment centers and improving healthcare infrastructure for advanced cell therapies.
Favorable reimbursement policies and insurance coverage improvements, gradually boosting patient access.
Strategic partnerships, collaborations & manufacturing scale-ups, reducing production costs and improving supply chain efficiency.
Growing awareness among oncologists and patients about CAR-T's superior therapeutic outcomes vs. conventional therapies.
Regional Insights:
North America, led by the U.S., commands the largest share of the global CAR-T cell therapy market, estimated at around 68% in recent assessments, driven by advanced healthcare infrastructure, robust biopharmaceutical R&D, high prevalence of hematologic cancers, FDA approvals, and favorable reimbursement policies.
Speak to Our Analyst and Get Customization in the report as per your requirements:https://www.datamintelligence.com/customize/us-car-t-cell-therapy-market?pratik
Key Players:
Novartis (Kymriah), Gilead/Kite Pharma (Yescarta, Tecartus), Bristol Myers Squibb (Breyanzi, Abecma), Johnson & Johnson (Carvykti), and Autolus (Aucatzyl).
Key Highlights (Top 5 Key Players) :
Novartis (Kymriah)
Novartis pioneered CAR-T with Kymriah (tisagenlecleucel), FDA-approved in 2017 for pediatric ALL and adult relapsed/refractory large B-cell lymphoma. Priced at $475,000 for ALL and $373,000 for lymphoma, it generated $536 million in 2022 sales despite a slight decline. Manufacturing occurs at Morris Plains, New Jersey, with contract support.
Gilead/Kite Pharma (Yescarta, Tecartus)
Gilead's Kite Pharma leads with Yescarta (axicabtagene ciloleucel), approved 2017 for large B-cell lymphoma, and Tecartus (brexucabtagene autoleucel), cleared for mantle cell lymphoma and adult ALL. Yescarta earned $1.16 billion in 2022, driving market dominance amid supply challenges.
Bristol Myers Squibb (Breyanzi, Abecma)
BMS offers Breyanzi for large B-cell lymphoma and Abecma for multiple myeloma, with Q2 sales of $39 million and $89 million respectively, projecting $500 million annually despite manufacturing bottlenecks. Both target BCMA/CD19, facing strong demand outstripping supply.
Johnson & Johnson (Carvykti)
J&J's Carvykti (ciltacabtagene autoleucel), partnered with Legend Biotech, gained FDA approval for relapsed multiple myeloma post-four therapies at $465,000 list price. In-house manufacturing ensures supply stability, competing directly with Abecma in a phased U.S. launch.
Autolus (Aucatzyl)
Autolus' Aucatzyl, FDA-approved in 2024 for relapsed B-cell ALL, boasts a favorable safety profile with low CRS (3% grade 3) and no REMS requirement. It targets 60% of U.S. patients initially via 30 centers, positioning as a tolerable CD19 CAR-T option.
Unlock 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription?pratik
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
✅ Competitive Landscape
✅ Sustainability Impact Analysis
✅ KOL / Stakeholder Insights
✅ Unmet Needs & Positioning, Pricing & Market Access Snapshots
✅ Market Volatility & Emerging Risks Analysis
✅ Quarterly Industry Report Updated
✅ Live Market & Pricing Trends
✅ Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release United States CAR-T Cell Therapy Market set for steady growth to US$ 9.85 billion by 2033 at a CAGR of 12.7% | Key Players:- Novartis, Gilead/Kite Pharma, Bristol Myers Squibb here
News-ID: 4325759 • Views: …
More Releases from DataM intelligence 4 Market Research LLP
Smart Factory Market to Reach US$ 290.6 Billion by 2033 at 12.6% CAGR; North Ame …
The Global Smart Factory Market reached US$ 112.4 billion in 2024 and is expected to reach US$ 290.6 billion by 2033, growing at a CAGR of 12.6% during the forecast period 2025-2033. Market growth is driven by increasing adoption of Industry 4.0 practices, rising demand for automation and operational efficiency, and growing integration of digital technologies across manufacturing industries.
Smart factories leverage advanced technologies such as the Industrial Internet of Things…
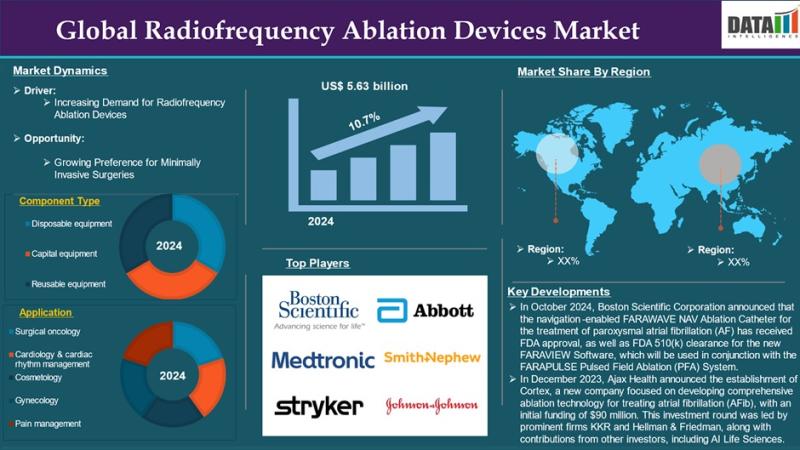
Radiofrequency Ablation Devices Market to Reach US$ 13.54 Billion by 2033 at 10. …
The Global Radiofrequency Ablation Devices Market reached US$ 5.63 billion in 2024 and is expected to reach US$ 13.54 billion by 2033, growing at a CAGR of 10.7% during the forecast period 2025-2033. Market growth is driven by the rising prevalence of chronic pain conditions and cancer, increasing preference for minimally invasive procedures, and advancements in ablation technologies.
Radiofrequency ablation devices are used to treat tumors, cardiac arrhythmias, and chronic pain…

Mobile Threat Defense (MTD) Market to Reach US$ 10.5 Billion by 2030, Growing at …
According to DataM Intelligence, the global Mobile Threat Defense (MTD) market reached US$ 2.2 billion in 2022 and is expected to reach US$ 10.5 billion by 2030, growing at a CAGR of 22.0% during the forecast period 2024-2031. This growth is propelled by the rapid surge in mobile malware, phishing, and ransomware attacks targeting enterprises, increasing adoption of BYOD and remote work policies, strong demand for cloud-based MTD solutions, rising…

Digital Identity Solutions Market to Reach US$ 189.92 Billion by 2033 at 20.45% …
The Global Digital Identity Solutions Market reached US$ 35.50 billion in 2023 and increased to US$ 42.45 billion in 2024, and is expected to reach US$ 189.92 billion by 2033, growing at a CAGR of 20.45% during the forecast period 2025-2033. Market growth is driven by the rising need for secure identity verification, increasing digitalization across industries, and growing concerns over identity fraud and data security.
Digital identity solutions enable the…
More Releases for FDA
DreaMed receives 5th FDA Clearance
TEL AVIV, Israel: DreaMed Diabetes LTD. ("DreaMed" or the "Company"), developer of the endo.digital Clinical Decision Support System announced today that it has received its 5th U.S Food and Drug Administration (FDA) clearance that expands the scope of AI enhanced treatment recommendations to patients on fixed meal insulin regimens. endo.digital is the first decision support system that has been cleared to assist healthcare providers in the management of diabetes…
FDA Compliant Blood Storage and Preservation
Accsense Monitoring System Automates Data Archive and Alarming
CAS DataLoggers provided the temperature alarming and monitoring system to a hospital blood bank looking to replace their old paper chart recorders as they became unreliable and spare parts were harder to find. For proper blood storage and preservation, the lab’s medical units needed to maintain storage temperatures between 2°C to 6°C (36°F to 43°F), given the perishability of blood components. The facility…
FDA grants orphan drug status to Vicore
US Food and Drug Administration has awarded Vicore Pharmaceuticals with orphan Drug designation for the treatment of Idiopathic Pulmonary Fibrosis (IPF). FDA’s Orphan Drug Designation program provides certain incentives for companies developing therapeutics to treat rare diseases or conditions, defined as those affecting less than 200,000 individuals in the U.S. A drug candidate and its sponsor must meet several key criteria in order to qualify for, and obtain, orphan drug…
New FDA Design Control Training Courses
Salt Lake City, Utah - February 23 2017 - Procenius Consulting is a medical device consulting firm specializing solely in medical device design controls regulation (21 CFR 820.30).
Announcing New Design Control Training Courses
Procenius Consulting has just launched two new training courses covering basic and advanced topics of medical device design control regulation. These courses focus on compliance, practical implementation and industry best practices techniques for developing or improving a…
fda online training
GRC Training Solutions provides end-to-end FDA compliance solutions for those companies who want to maximize security, minimize operational costs, improve staff productivity and stay on top of all their compliance documentation.
GRC Training Solutions boasts a team of experts and specialists who have a proven track record in working with the biotechnology, medical device, diagnostic and pharmaceutical fields. Our team will work with you closely and develop solutions that meet…
FDA online training
Description:
Device firms, establishments or facilities that are involved in the production and distribution of medical devices intended for use in the U.S are required to register annually. Most establishments that are required to register with the FDA are also required to list the devices that are made there and the activities that are performed on those devices. Initially, FDA issued a 28-page Proposed Rule that would amend its regulations regarding…