Press release
UK RNA Therapy Clinical Trials Market Outlook 2026-2036: Strategic Trends, Innovation Drivers & Growth Opportunities
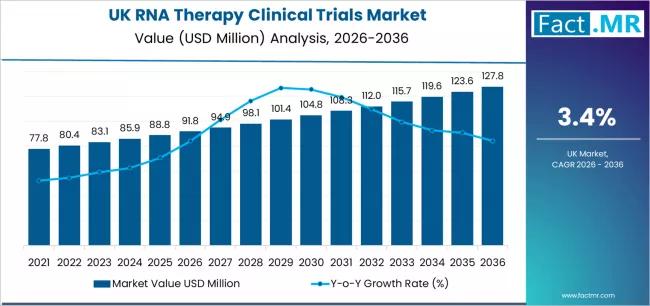
It is anticipated that the scope of RNA therapy clinical trials in UK will increase from USD 91.8 million in 2026 to around USD 127.8 million in 2036, representing a CAGR of 3.4% and a total growth of nearly 39.2%.Growing biotechnology capabilities, increased funding for genetic medicine, and broader use of precision-driven development paths all influence expansion.In the UK, strong biomedical research infrastructure, robust regulatory frameworks, and collaborative ecosystems are contributing to expanding clinical trial activity in RNA therapeutics across areas such as rare diseases, oncology, infectious diseases, and genetic disorders.
Key Takeaways from UK RNA Therapy Clinical Trials Industry Analysis
UK RNA Therapy Clinical Trials Sales Value (2026): USD 91.8 million
UK RNA Therapy Clinical Trials Forecast Value (2036): USD 127.8 million
UK RNA Therapy Clinical Trials Forecast CAGR: 3.4%
Leading End Use in UK RNA Therapy Clinical Trials Industry: Rare Diseases (22.3%)
Key Growth Regions in UK RNA Therapy Clinical Trials Industry: England, Scotland, Wales, and Northern Ireland
Regional Leadership: England holds the leading position in demand
Key Players in UK RNA Therapy Clinical Trials Industry: IQVIA Holdings Incorporated, ICON Public Limited Company, Laboratory Corporation of America Holdings, Charles River Laboratories International Incorporated, PAREXEL International Corporation, Syneos Health Incorporated, Medpace Holdings Incorporated, Pharmaceutical Product Development Incorporated, Novotech Health Holdings Private Limited, Veristat Limited Liability Company
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12704
Market Overview
RNA therapy clinical trials involve the investigation of safety, tolerability, efficacy, and optimal dosing of RNA-based therapeutic candidates in human subjects. These trials span multiple phases - from early-stage exploratory studies to late-stage confirmatory trials - and are critical for advancing novel RNA technologies through the clinical pipeline and toward regulatory approval.
The UK's well-established clinical research networks, centralized health data systems, and expertise in molecular medicine make it an attractive location for both domestic and international RNA therapy trial sponsors.
Key Demand Drivers
1. Scientific and Technological Advancements
Advancements in RNA biology and delivery systems fuel trial demand.
Improved mRNA vaccine platforms
Development of lipid nanoparticle and viral vector delivery
Enhanced stability and targeting of RNA molecules
These technologies expand potential indications and increase trial feasibility.
2. Success of RNA Therapeutics
High-profile clinical successes validate the modality.
Efficacy and safety of mRNA vaccines in public health
Approval of siRNA and ASO drugs for rare genetic conditions
Growing confidence among clinicians and sponsors
These successes catalyze broader investment and trial activity.
3. Strong Clinical Research Infrastructure
The UK has robust capabilities to support clinical development.
National Health Service (NHS) participation in research networks
Well-structured clinical trial governance and ethics oversight
Access to diverse patient populations
This infrastructure supports efficient trial execution and recruitment.
4. Regulatory Support and Innovation Incentives
Regulatory authorities in the UK are engaging with emerging therapies.
Adaptive regulatory pathways for novel modalities
Supportive guidance on RNA therapeutic development
Incentives for rare disease and advanced therapy research
Clearer regulatory frameworks reduce development uncertainty.
Market Segmentation Analysis
By RNA Therapy Type
mRNA Therapies:
Widely adopted due to success in vaccines and expanding into protein replacement and immuno-oncology.
siRNA Therapies:
Target gene silencing; growing interest in metabolic and genetic disorders.
Antisense Oligonucleotides (ASOs):
Modulate RNA splicing and gene expression in rare diseases.
RNA Editing and CRISPR-Based Modalities:
Emerging area with high potential and early clinical initiatives.
By Therapeutic Application
Oncology:
RNA-based immunotherapies and vaccine approaches against tumors.
Rare Genetic Disorders:
siRNA and ASO therapies targeting specific gene defects.
Infectious Diseases:
mRNA vaccines and therapeutic RNA candidates.
Cardiometabolic Conditions:
RNA approaches modulating lipid metabolism and chronic conditions.
Neurological Diseases:
ASO and siRNA strategies targeting neurodegenerative conditions.
By Trial Phase
Phase I Trials:
Early safety and dosage studies with growing activity.
Phase II Trials:
Proof-of-concept and efficacy studies in specific indications.
Phase III Trials:
Late-stage confirmatory trials leading to potential submissions for approval.
Activity is concentrated in early and mid-phase trials, reflecting a burgeoning pipeline.
End Users and Stakeholders
Biotechnology and Pharmaceutical Companies:
Primary sponsors initiating clinical programs.
Academic and Research Institutions:
Source of early innovation and investigator-initiated trials.
Contract Research Organizations (CROs):
Key partners managing operational aspects of trials.
Specialized Clinical Trial Centers:
Provide site infrastructure and patient recruitment.
Patient Advocacy Groups:
Support rare disease trial awareness and enrollment.
Regional Trends within the UK
England (including London and Oxford-Cambridge corridor):
Highest concentration of RNA research and clinical sites.
Scotland and Wales:
Growing clinical research activity supported by regional academic centers.
Northern Ireland:
Emerging participation with links to broader UK trial networks.
Geographic distribution reflects research expertise, infrastructure, and institutional presence.
Challenges and Market Restraints
1. Biological Delivery and Safety Complexities
Efficient and safe delivery of RNA remains challenging
Need to minimize off-target effects and immune activation
2. Funding and Development Costs
High upfront investment for RNA clinical programs
Need for sustained funding from investors and sponsors
3. Regulatory and Ethical Considerations
Evolving regulatory expectations for novel modalities
Ethical requirements around genetic and personalized therapies
Emerging Trends and Opportunities
1. Personalized RNA Therapeutics
Tailored mRNA and ASO therapies targeting individual genotypes
Expanded scope in precision medicine
2. Combination RNA Approaches
Integration with immunotherapy and targeted agents
Synergistic strategies to enhance efficacy
3. Digital and Real-World Evidence Integration
Use of NHS health data for patient selection and outcomes tracking
Digital recruitment and monitoring platforms
4. Expansion in Rare and Underserved Conditions
Focus on rare genetic and metabolic diseases
Strong clinical and patient advocacy support
Competitive Landscape Overview
Stakeholders are focusing on:
Investment into RNA platform technologies
Strategic partnerships between biotech and academic hubs
Early engagement with regulatory authorities
Demonstration of safety and clinical benefit
Competitive advantage is driven by scientific expertise, trial execution capability, and innovation in delivery systems.
Future Outlook
The demand for RNA therapy clinical trials in the UK is expected to sustain strong growth through 2035, anchored by scientific breakthroughs, supportive research infrastructure, and expanding therapeutic indications. While challenges remain in delivery technologies and regulatory clarity, the combination of NHS-enabled research networks, biotech innovation, and patient engagement positions the UK as a significant hub for RNA clinical development.
Browse Full Report: https://www.factmr.com/report/united-kingdom-rna-therapy-clinical-trials-market
Purchase Full Report for Detailed Insights
For access to full forecasts, regional break-outs, product- and application-level analysis, company share details, and emerging trend assessments, you can purchase the complete report: https://www.factmr.com/checkout/12704
Have specific requirements or need assistance on report pricing or have a limited budget? Please contact sales@factmr.com
Related Reports:
Demand for Doxorubicin in USA: https://www.factmr.com/report/united-states-doxorubicin-market
Demand for Cell Sorting in USA: https://www.factmr.com/report/united-states-cell-sorting-market
Demand for Hospital Acquired Infections Therapeutics in UK: https://www.factmr.com/report/united-kingdom-hospital-acquired-infections-therapeutics-market
Demand for Cancer Tumor Profiling in UK: https://www.factmr.com/report/united-kingdom-cancer-tumor-profiling
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release UK RNA Therapy Clinical Trials Market Outlook 2026-2036: Strategic Trends, Innovation Drivers & Growth Opportunities here
News-ID: 4319413 • Views: …
More Releases from Fact.MR
Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
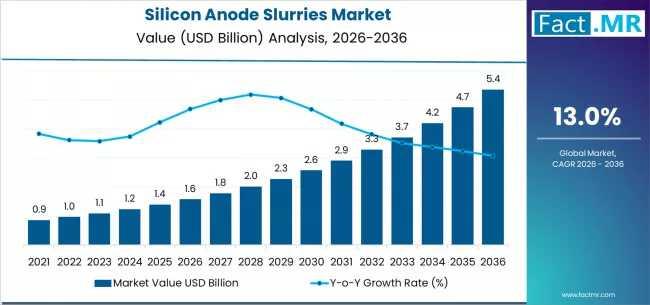
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…

Silicon Anode Slurries Market Forecast 2026-2036: Market Size, Share, Competitiv …
The global silicon anode slurries market is set for significant expansion between 2026 and 2036, fueled by the rising adoption of high-energy-density lithium-ion batteries across electric vehicles (EVs), consumer electronics, and grid-scale energy storage. As battery manufacturers increasingly transition from graphite to silicon-enhanced anodes, the demand for high-performance, scalable silicon anode slurries is projected to grow sharply.
To access the complete data tables and in-depth insights, request a Discount On The…
Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
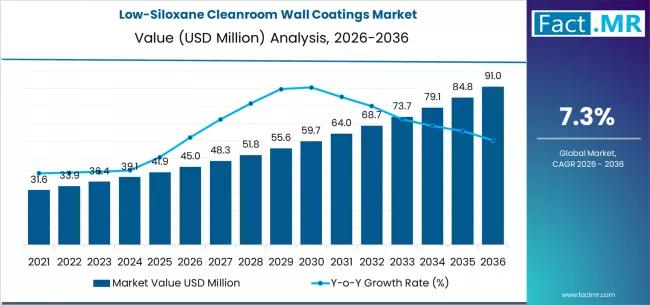
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…

Low-Siloxane Cleanroom Wall Coatings Market Deep-Dive 2026-2036: Strategic Forec …
The low-siloxane cleanroom wall coatings market is poised for steady growth over the next decade, driven by rising contamination-control requirements across semiconductor, pharmaceutical, biotechnology, and precision manufacturing industries. These coatings are specifically engineered to minimize siloxane outgassing and volatile organic compound emissions, helping maintain ultra-clean environments where even trace contamination can disrupt production quality.
By 2036, the market for low-siloxane cleanroom wall coatings is expected to grow to USD 91.04 million.…
More Releases for RNA
CD Formulation Launches Custom Circular RNA Synthesis Service to Accelerate RNA …
CD Formulation introduces a customizable circRNA synthesis service, delivering high-quality, stable circRNAs for therapeutics, vaccines, and gene research, supported by advanced design and QC processes.
CD Formulation, a leading provider of advanced small nucleic acid synthesis [https://www.formulationbio.com/nucleic-acid/custom-small-nucleic-acid-synthesis.html] solutions, is proud to announce the launch of its fully customizable circular RNA (circRNA) synthesis service. This new service addresses the growing need for stable, non-immunogenic RNA molecules for therapeutic development, vaccine research, and…
Self-Amplifying RNA Synthesis Market Gains Traction as Biotech Firms Embrace Sca …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " Self-Amplifying RNA Synthesis Market- (By Product & Service (Products (Enzymes & Reagents, Premade saRNA, Others), Custom Synthesis Services), By Application (Therapeutics Development (Oncology, Infectious Diseases, Others), Biomedical Research), By End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic,…
RNA Extraction and RNA Purification Market: Growth, Trends & Competitive Landsca …
The global RNA Extraction and RNA Purification Market is expected to grow at 6.3% CAGR from 2025 to 2032.
This Market Report is the result of extensive research and analysis conducted by our team of experienced market researchers through -
• 70% efforts of Primary Research
• 15% efforts of Secondary Research
• 15% efforts from the subscription to Paid database providing industry overview, macro and micro economics factors, and financials of private limited…
RNA Targeting Small Molecules Therapeutics Market: Exponential Growth with Risin …
Estimations Predict a CAGR of 29.8% by 2029 in Global RNA Targeting Small Molecules Therapeutics Market Boosted by Precision Medicine, RNA Biomarker Identification and RNA Genetic Manipulation
What Is The Projected Market Size of The Global RNA Targeting Small Molecules Therapeutics Market And Its Growth Rate?
• The market will grow from $6.1 billion in 2024 to $7.87 billion in 2025 at a compound annual growth rate (CAGR) of 28.9%.
• Expected exponential…
Global DNARNA Extraction Kit Market by Type (Cell-free DNA (cfDNA), Sequence-spe …
"DNARNA Extraction Kit Market" is segmented by Company, Region (country), By Type, Application, stakeholders and other participants. This report provides an analysis of revenue and forecast across Type and Application segments for 2023-2032.
The market for DNARNA Extraction Kits has been thoroughly researched via primary and secondary sources to produce this research study. Along with a competitive analysis of the market, segmented by application, type, and geographical trends, it offers a…
Cancer RNA Expression Market to Reap Excessive Revenues by 2028(By sequencing te …
Worldwide cancer is one of the leading cause of death and effective way of treating it still looks unaccomplished in most parts of the world. The factors which influence the successful treatment of cancer are different depending on the stage of diagnosis, treatment availability and availability of trained healthcare professionals coupled with high economic burden of the disease. The gene expression of cancerous cells varies by cancer type and may…