Press release
Multiple Myeloma Clinical Trial Pipeline Gains Momentum: 75+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
DelveInsight's "Multiple Myeloma Pipeline Insights 2025" report provides comprehensive insights about 75+ Multiple Myeloma Companies and 80+ pipeline drugs in the Multiple Myeloma pipeline landscape. It covers the Multiple Myeloma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Multiple Myeloma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore the comprehensive insights by DelveInsight and stay ahead in understanding the Multiple Myeloma Treatment Landscape. Click here to read more @ Multiple Myeloma Pipeline Outlook [https://www.delveinsight.com/sample-request/multiple-myeloma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Multiple Myeloma Pipeline Report
* On December 15, 2025- Pfizer initiated a study will compare the experiences of people receiving elranatamab to those people receiving a combination therapy. This will help learn about the safety and how effective elranatamab is. The purpose of this study is to learn about the study medicine called elranatamab.This study aims to compare elranatamab to other medicines for the treatment of MM (a type of cancer).
* On December 12, 2025- European Myeloma Network B.V. announced a study is researching an experimental drug called linvoseltamab. The study is focused on participants with newly diagnosed multiple myeloma (NDMM) who are ineligible for autologous stem cell transplantation (transplant-ineligible). The main purpose of this study is to compare the effect and safety of linvoseltamab with the effect and safety of the standard treatment.
* On December 10, 2025, AbbVie conducted a phase 1/2, Open-Label, Platform Study to Evaluate Safety and Efficacy of Etentamig Monotherapy or Etentamig Combinations in Subjects With Multiple Myeloma. This study is broken into 4 substudies and each substudy consists of a dose escalation phase and dose expansion phase. Participants will receive escalating doses of etentamig alone or in combination with daratumumab and lenalidomide (DR), carfilzomib and dexamethasone (Kd) or lenalidomide (R).
* DelveInsight's Multiple Myeloma pipeline report depicts a robust space with 75+ Multiple Myeloma companies working to develop 80+ pipeline therapies for Multiple Myeloma treatment.
* The leading Multiple Myeloma Companies such as CASI Pharmaceuticals, Carsgen Therapeutics, Cartesian Therapeutics, Gracell Biotechnology Shanghai Co., Ltd., Sorrento Therapeutics, TeneoOne, Karyopharma Therapeutics, Arcellx, Poseida Therapeutics, Ichnos Sciences, Nerviano Medical Sciences, Bristol Myers Squib, Ascentage Pharma, Ionis Pharmaceuticals, Chongqing Precision Biotech Co., Ltd., CRISPR Therapeutics, AstraZeneca, IGM Biosciences, Novartis, GlaxoSmithKline, Innovent Biologics, Keymed Biociences, Starton Therapeutics, Takeda, Fate Therapeutics, Gilead Sciences, Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., Janssen Pharmaceutical, Nanjing IASO Biotechnology Co., Ltd., GPCR Therapeutics, Chimerix , and others.
* Promising Multiple Myeloma Pipeline Therapies such as TNB-383B, belantamab mafodotin, Pembrolizumab, Melphalan flufenamide (Melflufen), Dexamethasone, Pomalidomide, Venetoclax, Bortezomib, BT062, Selinexor, Lenalidomide, Methylprednisolone , and others.
Discover groundbreaking developments in Multiple Myeloma therapies! Gain in-depth knowledge of key Multiple Myeloma clinical trials, emerging drugs, and market opportunities @ Multiple Myeloma Clinical Trials Assessment [https://www.delveinsight.com/sample-request/multiple-myeloma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Multiple Myeloma Overview
Myelodysplastic syndrome (MDS) is a heterogeneous group of hematologic neoplasms classically described as a clonal disorder of hematopoietic stem cells leading to dysplasia and ineffective hematopoiesis in the bone marrow. Some patients with MDS may have a transformation into acute myeloid leukemia (AML). MDS is usually diagnosed in older patients over the age of 65. Clinical manifestations include a decrease in the number of red blood cells (RBC), platelets, and white blood cells (WBC). The disease course is variable.
Multiple myeloma Emerging Drugs Profile
* Zevorcabtagene Autoleucel: Carsgen Therapeutics
Zevorcabtagene Autoleucel (Zevor-cel, R&D code: CT053) is an autologous fully human CAR T-cell product candidate against B-cell maturation antigen (BCMA) for the treatment of relapsed/refractory multiple myeloma (R/R MM). In October 2022, China National Medical Products Administration (NMPA) accepted the New Drug Application (NDA) and has granted the priority review for zevor-cel. Zevor-cel is expected to be approved by the NMPA for the treatment of R/R MM at the end of 2023 or the beginning of 2024. In January 2023, CARsgen and Huadong Medicine entered into a collaboration agreement for the commercialization of CARsgen's lead drug candidate, zevor-cel, in mainland China. Since reaching the agreement, teams from CARsgen and Huadong Medicine have been working together closely to implement this collaboration and prepare for the approval and commercialization of zevor-cel in China.
* Descartes 08: Cartesian Therapeutics
Descartes-08 is an autologous BCMA-targeting RNA-modified CAR T-cell therapy. Descartes-08 is engineered by mRNA transfection to express anti-BCMA CAR for a defined length of time. Descartes-08 express anti-BCMA CAR for 1 week, limiting risk of uncontrolled proliferation; produce inflammatory cytokines in response to myeloma target cells; and are highly cytolytic against myeloma cells regardless of presence of myeloma-protecting bone marrow stromal cells, exogenous a proliferation-inducing ligand, or drug resistance including IMiDs. The magnitude of cytolysis correlates with anti-BCMA CAR expression duration, indicating a temporal limit in activity. In early-stage clinical studies, Descartes-08 has been safe and well-tolerated in patients with MG and multiple myeloma. Currently, the drug is in the Phase II stage of its development for the treatment of Multiple Myeloma.
* GC012F: Gracell Biotechnology Shanghai Co., Ltd.
GC012F is Gracell's FasTCAR-enabled BCMA/CD19 dual-targeting autologous CAR-T cell therapy, which aims to transform cancer and autoimmune disease treatment by driving fast, deep and durable responses with improved safety profile. GC012F is currently being evaluated in clinical studies in multiple hematological cancers as well as autoimmune diseases, and has demonstrated a consistently strong efficacy and safety profile. Gracell has initiated a Phase 1b/2 trial evaluating GC012F for the treatment of relapsed/refractory multiple myeloma in the United States and a Phase 1/2 clinical trial in China is to be commenced imminently.
* CID-103: CASI Pharmaceuticals
CID-103 is a fully human IgG1 anti-CD38 monoclonal antibody that recognizes a unique epitope on CD38. It was engineered to have strong activity against CD38 malignant cells and to reduce certain safety issues observed with existing treatments. Preclinical data of CID-103 demonstrates enhanced activity against a broad array of malignancies which express CD38 and demonstrates a better preclinical safety profile when compared to other CD38 mAbs. These attributes offer the potential for accelerated development and regulatory review, including rapid advancement into earlier lines of therapy. Currently, the drug is in the Phase I stage of its development for the treatment of Multiple Myeloma.
* STI-1492: Sorrento Therapeutics
STI-1492 is a therapeutic candidate developed by Sorrento Therapeutics for the treatment of relapsed or refractory multiple myeloma. It is an allogeneic, off-the-shelf therapy that involves the administration of Anti-CD38 A2 Dimeric Antigen Receptor T (DAR-T) cells through a single intravenous infusion. The therapy is currently being evaluated in a phase 1b, open-label, dose-escalation study involving subjects with relapsed or refractory multiple myeloma. CD38 is a transmembrane glycoprotein present on various immune cells and hematologic malignancies, and its expression has been associated with poor prognosis. Currently, the drug is in the Phase I stage of its development for the treatment of Multiple Myeloma.
The Multiple Myeloma Pipeline report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Multiple Myeloma with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Multiple Myeloma Treatment.
* Multiple Myeloma Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Multiple Myeloma Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Multiple Myeloma market
Stay informed about the Multiple Myeloma pipeline trends! Uncover critical updates on therapeutic innovations and their potential impact on patients and the healthcare industry @ Multiple Myeloma Unmet Needs [https://www.delveinsight.com/sample-request/multiple-myeloma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Multiple Myeloma Companies
CASI Pharmaceuticals, Carsgen Therapeutics, Cartesian Therapeutics, Gracell Biotechnology Shanghai Co., Ltd., Sorrento Therapeutics, TeneoOne, Karyopharma Therapeutics, Arcellx, Poseida Therapeutics, Ichnos Sciences, Nerviano Medical Sciences, Bristol Myers Squib, Ascentage Pharma, Ionis Pharmaceuticals, Chongqing Precision Biotech Co., Ltd., CRISPR Therapeutics, AstraZeneca, IGM Biosciences, Novartis, GlaxoSmithKline, Innovent Biologics, Keymed Biociences, Starton Therapeutics, Takeda, Fate Therapeutics, Gilead Sciences, Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., Janssen Pharmaceutical, Nanjing IASO Biotechnology Co., Ltd., GPCR Therapeutics, Chimerix and others.
Multiple myeloma pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Multiple Myeloma Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
Transform your understanding of the Multiple Myeloma Pipeline! See the latest progress in drug development and clinical research @ Multiple Myeloma Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/multiple-myeloma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Multiple Myeloma Pipeline Report
* Coverage- Global
* Multiple Myeloma Companies- CASI Pharmaceuticals, Carsgen Therapeutics, Cartesian Therapeutics, Gracell Biotechnology Shanghai Co., Ltd., Sorrento Therapeutics, TeneoOne, Karyopharma Therapeutics, Arcellx, Poseida Therapeutics, Ichnos Sciences, Nerviano Medical Sciences, Bristol Myers Squib, Ascentage Pharma, Ionis Pharmaceuticals, Chongqing Precision Biotech Co., Ltd., CRISPR Therapeutics, AstraZeneca, IGM Biosciences, Novartis, GlaxoSmithKline, Innovent Biologics, Keymed Biociences, Starton Therapeutics, Takeda, Fate Therapeutics, Gilead Sciences, Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., Janssen Pharmaceutical, Nanjing IASO Biotechnology Co., Ltd., GPCR Therapeutics, Chimerix , and others.
* Multiple Myeloma Pipeline Therapies- TNB-383B, belantamab mafodotin, Pembrolizumab, Melphalan flufenamide (Melflufen), Dexamethasone, Pomalidomide, Venetoclax, Bortezomib, BT062, Selinexor, Lenalidomide, Methylprednisolone, and others.
* Multiple Myeloma Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Multiple Myeloma Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Stay Ahead in Oncology Research-Access the Full Multiple Myeloma Pipeline Analysis Today! @ Multiple Myeloma Drugs and Companies [https://www.delveinsight.com/sample-request/multiple-myeloma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Multiple myeloma: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Multiple myeloma- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Venetoclax: AbbVie
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* PHE885: Novartis
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* ONC 201: Oncoceutics
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* TNB 383B: TeneoBio
* Drug profiles in the detailed report.....
* Inactive Products
* Multiple myeloma Key Companies
* Multiple myeloma Key Products
* Multiple myeloma- Unmet Needs
* Multiple myeloma- Market Drivers and Barriers
* Multiple myeloma- Future Perspectives and Conclusion
* Multiple myeloma Analyst Views
* Multiple myeloma Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=multiple-myeloma-clinical-trial-pipeline-gains-momentum-75-companies-lead-the-charge-in-pioneering-new-treatments-delveinsight]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/multiple-myeloma-pipeline-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Multiple Myeloma Clinical Trial Pipeline Gains Momentum: 75+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight here
News-ID: 4318062 • Views: …
More Releases from ABNewswire
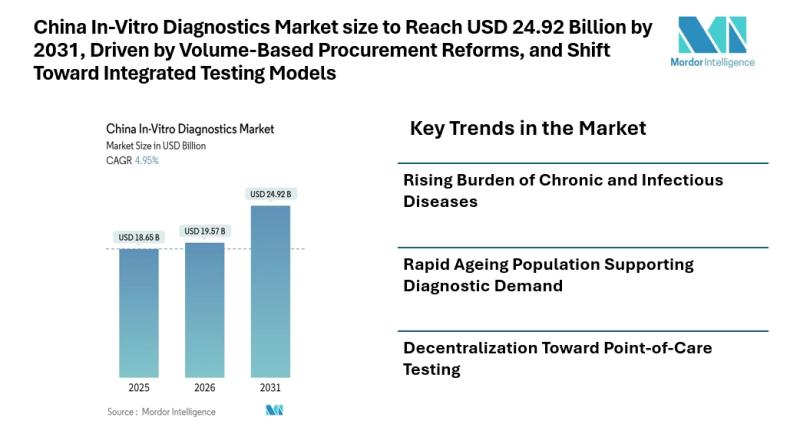
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Multiple
Mango AI's Multiple Face Swap Video Tool Changes Multiple Faces Instantly
In today's digital world, creating eye-catching and unique content is essential for standing out. The ability to swap multiple faces in a single video opens up creative possibilities, whether for personalized content, comedy or parody videos, or engaging social media posts. Mango AI, developed by Mango Animate, is excited to announce the launch of its advanced multiple face swap video (https://mangoanimate.com/ai/multiple-face-swap-video) tool, which is designed to simplify the creation of…
Multiple Sclerosis Drugs Market
Global Multiple Sclerosis Drugs Market Size research report offers in-depth assessment of revenue growth, market definition, segmentation, industry potential, influential trends for understanding the future outlook and current prospects for the market.
Get a Sample Copy of the Report at - https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/100386
Multiple Sclerosis (MS) is an immune-mediated disease affecting the central nervous system. It is characterized by demyelination, inflammation, degenerative changes such as progressive brain and spinal cord atrophy and neuroaxonal…
Massive Multiple Input Multiple Output (MIMO) Market May Set New Growth Story
A Latest intelligence report published by MR Forecast with title "Global Massive Multiple Input Multiple Output (MIMO) Market Outlook to 2032. A detailed study accumulated to offer Latest insights about acute features of the Massive Multiple Input Multiple Output (MIMO) market. This report provides a detailed overview of key factors in the Global Massive Multiple Input Multiple Output (MIMO) Market and factors such as driver, restraint, past and current trends,…
Multiple Sclerosis Drugs Market - Progressing Towards a Cure: Advancing Multiple …
Newark, New Castle, USA: The "Multiple Sclerosis Drugs Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Multiple Sclerosis Drugs Market: https://www.growthplusreports.com/report/multiple-sclerosis-drugs-market/7983
This latest report researches the industry structure,…
Multiple Sclerosis Drugs Market - Empowering Resilience, Embracing Possibilities …
Newark, New Castle, USA - new report, titled Multiple Sclerosis Drugs Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Multiple Sclerosis Drugs market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Multiple Sclerosis Drugs market. The report offers an overview of…
Massive MIMO (Multiple-input multiple-output) Market 2022-2028 | Detailed Report
The market report delivers an all-inclusive analysis of the market structure along with a forecast of the various segments and sub-segments of the Massive MIMO (Multiple-input multiple-output) industry. This wide-ranging market research report acts as a backbone for the success of business in any niche. The Massive MIMO (Multiple-input multiple-output) market survey report has been prepared by conducting market research in a systematic manner. Moreover, the Massive MIMO (Multiple-input multiple-output)…
