Press release
AI in Healthcare Regulatory Affairs Market Size Forecast to USD 6.5 Billion by 2035 with a Focus on Automated Compliance and Faster Drug Approvals - Analysis by Transparency Market Research
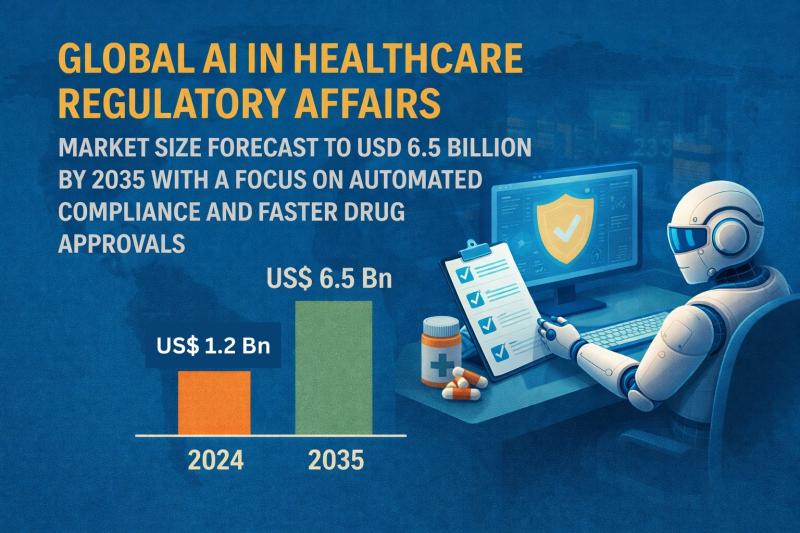
AI in Healthcare Regulatory Affairs Market Size Outlook 2035The global AI in healthcare regulatory affairs market was valued at US$ 1.2 billion in 2024. It is projected to expand at a robust CAGR of 16.7% from 2025 to 2035, reaching US$ 6.5 billion by 2035. This strong growth reflects the increasing adoption of artificial intelligence to streamline regulatory processes, ensure compliance, reduce approval timelines, and manage complex regulatory data across the healthcare ecosystem.
👉 Get your sample market research report copy today@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86874
Market Overview
Healthcare regulatory affairs involve managing compliance with regulations governing drug development, clinical trials, medical devices, biologics, and digital health solutions. Traditionally, regulatory processes are time-consuming, document-intensive, and prone to human error.
AI is transforming regulatory affairs by enabling:
• Automated document generation and submission
• Real-time regulatory intelligence and monitoring
• Predictive analytics for approval timelines and compliance risks
• Natural language processing (NLP) for regulatory content analysis
Pharmaceutical companies, biotechnology firms, medical device manufacturers, and CROs are increasingly integrating AI solutions to accelerate time-to-market, improve accuracy, and reduce regulatory bottlenecks. Regulatory authorities are also gradually embracing digital tools, further supporting market expansion.
Key Market Growth Drivers
1. Rising Regulatory Complexity
Global healthcare regulations are becoming increasingly complex and region-specific. AI tools help organizations interpret evolving regulations, track changes, and maintain compliance across multiple geographies.
2. Need to Reduce Drug Approval Timelines
Pharmaceutical and biotech companies face intense pressure to speed up regulatory submissions. AI-powered platforms automate data validation, dossier preparation, and gap analysis, significantly reducing approval cycles.
3. Growing Volume of Regulatory Data
Clinical trials, real-world evidence, safety reports, and post-market surveillance generate massive data volumes. AI enables efficient data processing, classification, and insight generation.
4. Cost Reduction and Operational Efficiency
Automation of repetitive regulatory tasks lowers operational costs and minimizes dependency on manual resources, driving AI adoption across large and mid-sized healthcare companies.
5. Increasing Adoption of Digital Health and AI-Based Therapies
The rise of digital therapeutics, AI-driven diagnostics, and software as a medical device (SaMD) requires continuous regulatory oversight, creating demand for AI-powered regulatory tools.
Analysis of Key Players - Key Player Strategies
Key players in the AI healthcare regulatory affairs market are focused on platform innovation, strategic collaborations, and expanding regulatory intelligence capabilities.
1. Product Innovation and Platform Development
• AI-driven regulatory submission management systems
• NLP-based tools for labeling, variation management, and compliance tracking
• Predictive analytics for regulatory approval outcomes
2. Strategic Partnerships
• Collaborations with pharmaceutical companies, CROs, and regulatory consultancies
• Partnerships with cloud providers and data analytics firms to enhance scalability
3. Geographic Expansion
• Expansion into North America and Europe, where regulatory scrutiny is high
• Growing presence in Asia-Pacific to support expanding pharma manufacturing hubs
4. Focus on Compliance and Data Security
• Integration of GxP compliance, cybersecurity, and data governance frameworks
• Development of explainable AI models to meet regulatory transparency requirements
Analysis of Key Players in the AI in Healthcare Regulatory Affairs Market
Companies operating in the AI in healthcare regulatory affairs market are increasingly focusing on strategic collaborations, platform integration, and AI-driven automation to enhance regulatory compliance and operational efficiency. These firms are investing in predictive analytics, cloud-based platforms, and advanced regulatory intelligence tools. At the same time, they are expanding their service portfolios and geographic presence to deliver end-to-end regulatory support across global markets.
Leading players in the global AI in healthcare regulatory affairs market include
• Clarivate
• IQVIA Inc.
• Wipro
• Freyr
• Innoplexus
• Zenovel
• Indegene
• RegDesk, Inc.
• CELEGENCE
• Rimsys
• DDi.
• DXC Technology Company
• Ketryx Corporation.
Each of these companies is profiled in the AI in healthcare regulatory affairs market research report based on parameters such as company overview, financial performance, business strategies, product portfolio, business segments, and recent developments.
👉 Discuss Implications for Your Industry Request Sample Research Report PDF@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86874
Key Developments in the AI in Healthcare Regulatory Affairs Market
• September 2025: Elsevier launched PharmaPendium AI, a generative AI assistant designed to support regulatory intelligence across drug development. The solution enhances how regulatory affairs professionals, as well as preclinical and clinical researchers, search and access information from regulatory publications issued by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
• August 2025: Clarivate Plc introduced an AI-driven Regulatory Assistant within its Cortellis Regulatory Intelligence platform. This feature is designed to simplify and accelerate navigation of complex and evolving global regulatory requirements. Developed based on customer feedback and piloted with industry partners, the beta solution addresses real-world needs of regulatory professionals across biopharma, medtech, and research institutions.
Market Challenges & Opportunities
Challenges
1. Regulatory Acceptance of AI
Regulators require transparency and explainability in AI models, which may slow adoption.
2. Data Privacy and Security Concerns
Handling sensitive patient and clinical data requires robust cybersecurity and compliance frameworks.
3. High Implementation Costs
Initial investment in AI platforms and integration with legacy systems can be expensive for smaller organizations.
4. Skill Gaps
Shortage of professionals skilled in both regulatory affairs and AI technologies poses adoption challenges.
Opportunities
1. Growing Demand from Mid-Sized Pharma and Biotech Firms
AI democratizes regulatory expertise, enabling smaller companies to compete effectively.
2. Integration with Real-World Evidence (RWE)
AI-driven regulatory platforms can analyze RWE to support post-market approvals and label expansions.
3. Emerging Markets Regulatory Digitalization
Developing countries are modernizing regulatory systems, creating new growth opportunities.
4. AI-Enabled Continuous Compliance
Shift from reactive to proactive, real-time regulatory compliance offers long-term value creation.
Investment Landscape and ROI Outlook
The AI in healthcare regulatory affairs market presents high-growth investment potential, supported by digital transformation across life sciences.
Investment Strengths
• Strong CAGR of 16.7% through 2035
• Increasing regulatory scrutiny across healthcare sectors
• Rising adoption of AI across the drug development lifecycle
• Growing demand for faster and more compliant market access
ROI Outlook
Investments in AI regulatory platforms, SaaS-based solutions, and data analytics tools are expected to deliver high ROI, particularly for vendors offering scalable, compliant, and explainable AI solutions. Early investments in Asia-Pacific and emerging markets may yield above-average returns.
Market Segmentations
By Component
• Software
• Services
By Deployment Mode
• Cloud-Based
• On-Premise
By Application
• Regulatory Submission Management
• Regulatory Intelligence
• Labeling and Artwork Management
• Compliance and Audit Management
• Post-Market Surveillance
By End User
• Pharmaceutical Companies
• Biotechnology Companies
• Medical Device Manufacturers
• CROs
• Regulatory Consulting Firms
By Region
• North America
• Europe
• Asia-Pacific
• Latin America
• Middle East & Africa
👉 To buy this comprehensive market research report, click here to inquire@ https://www.transparencymarketresearch.com/checkout.php?rep_id=86874<ype=S
Why Buy This Report?
✔ In-depth market forecast through 2035
✔ Detailed analysis of AI adoption in regulatory workflows
✔ Competitive landscape with key player strategies
✔ Insights into regulatory challenges and digital transformation trends
✔ Segmentation analysis to identify high-growth opportunities
✔ Strategic guidance for investors, technology providers, and healthcare companies
FAQs
1. What is the projected market size by 2035?
The market is expected to reach US$ 6.5 billion by 2035.
2. What is the CAGR from 2025 to 2035?
The market is projected to grow at a CAGR of 16.7%.
3. Which segment dominates the market?
Regulatory submission management software currently dominates due to high automation demand.
4. Which region leads the market?
North America leads due to advanced healthcare IT infrastructure and regulatory complexity.
5. What are the key trends?
Key trends include AI-powered regulatory intelligence, cloud-based platforms, NLP-driven automation, and real-time compliance monitoring.
More Trending Research Reports-
• Wearable Healthcare Devices Market - https://www.transparencymarketresearch.com/wearable-healthcare-devices-market.html
• PCR & Real-time PCR Molecular Diagnostics Market - https://www.transparencymarketresearch.com/pcr-realtime-pcr-molecular-diagnostics-market.html
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
Contact Us
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Blog: https://tmrblog.com
Email: sales@transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release AI in Healthcare Regulatory Affairs Market Size Forecast to USD 6.5 Billion by 2035 with a Focus on Automated Compliance and Faster Drug Approvals - Analysis by Transparency Market Research here
News-ID: 4317788 • Views: …
More Releases from Transparency Market Research Pvt Ltd

Hospital Hygiene Management Market Size Forecast to USD 32.7 Billion by 2036 wit …
Hospital Hygiene Management Market Outlook to 2036
The global hospital hygiene management market was valued at US$ 11.1 billion in 2025 and is projected to reach US$ 32.7 billion by 2036, expanding at a robust CAGR of 10.3% from 2026 to 2036. The strong growth trajectory reflects rising awareness of infection prevention, patient safety, and regulatory compliance across healthcare facilities worldwide.
👉 Get your sample market research report copy today@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86942
Market Overview
Hospital…
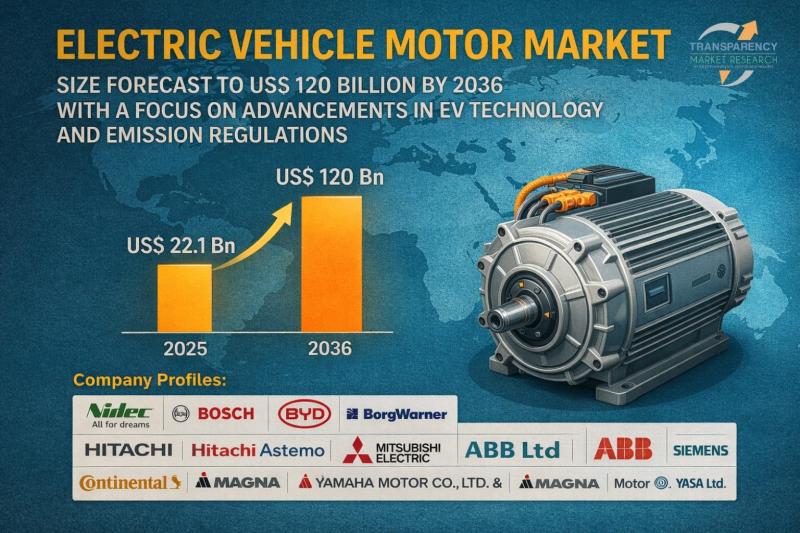
Electric Vehicle Motor Market Size Forecast to USD 120 Billion by 2036 with a Fo …
Electric Vehicle Motor Market Outlook to 2036
The global electric vehicle (EV) motor market was valued at US$ 22.1 billion in 2025. It is projected to grow at a CAGR of 16.0% from 2026 to 2036, reaching US$ 120 billion by 2036. The growth is primarily driven by stringent emissions regulations, government incentives for electric mobility, and rapid advancements in EV motor and battery technologies.
👉 Get your sample market research report…
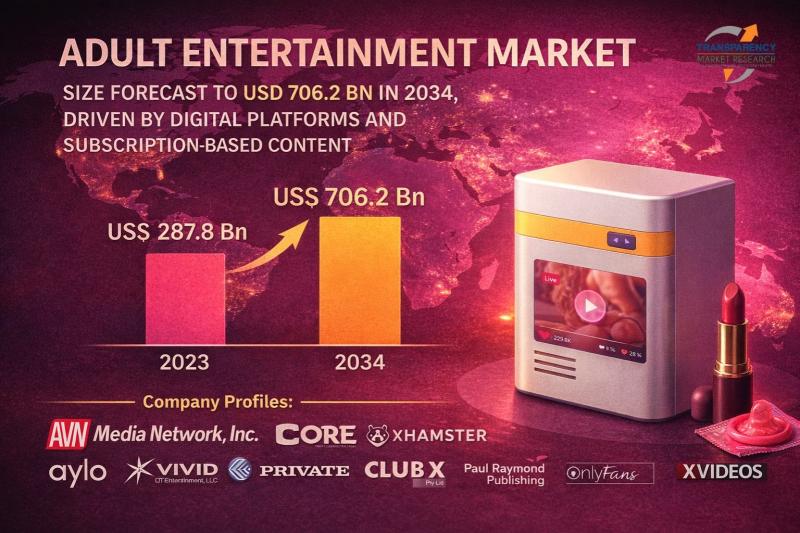
Adult Entertainment Market Size Forecast to USD 706.2 Billion by 2034, Driven by …
Adult Entertainment Market Outlook 2034
The global adult entertainment market was valued at US$ 287.8 billion in 2023. The industry is projected to expand at a robust CAGR of 8.6% from 2024 to 2034, reaching an estimated US$ 706.2 billion by the end of 2034. Market growth is driven by rapid digitalization, increasing internet penetration, changing social attitudes, and the monetization of personalized adult content across platforms.
👉 Get your sample market…
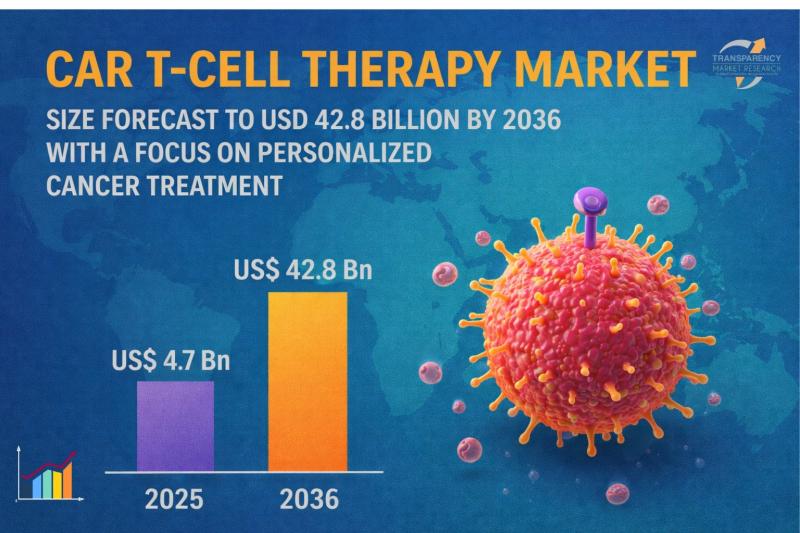
CAR T-cell Therapy Market Size Forecast to USD 42.8 Billion by 2036 with a Focus …
CAR T-cell Therapy Market Outlook to 2036
The global CAR T-cell therapy market was valued at US$ 4.7 billion in 2025 and is projected to reach US$ 42.8 billion by 2036, expanding at a robust CAGR of 22.3% from 2026 to 2036. This exceptional growth trajectory is primarily driven by rapid advancements in genetic engineering technologies, rising demand for personalized medicine, and increasing approvals of CAR T-cell therapies for hematological malignancies.
👉…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…