Press release
Executive Report: Future of the USA Controlled Release Drug Delivery Indystry- Key Drivers, Disruption Signals & Industry Scenarios
Demand for controlled release drug delivery in the USA is expected to expand by USD 49.32 billion over the course of the forecast period, from USD 28.16 billion in 2025 to over USD 77.48 billion by 2035. Demand is expected to grow at a compound annual growth rate (CAGR) of 10.7% between 2025 and 2035, translating into a total growth of 175.1%.In the U.S., where chronic diseases are highly prevalent and healthcare costs remain a key concern, controlled release formulations are becoming an essential part of modern drug development. These systems support long-term disease management, reduce dosing frequency, and enhance patient adherence-factors that are critically important in both outpatient and hospital settings.
Quick Market Overview (2025-2035)
USA Controlled Release Drug Delivery Sales Value (2025): USD 28.16 billion
USA Controlled Release Drug Delivery Forecast Value (2035): USD 77.48 billion
USA Controlled Release Drug Delivery Forecast CAGR: 10.7%
Leading Technology in USA Controlled Release Drug Delivery Industry: Targeted Delivery (26.3%)
Key Growth Regions in USA Controlled Release Drug Delivery Industry: West, Northeast, South, Midwest
Regional Leadership: West holds the leading position in demand
Key Players in USA Controlled Release Drug Delivery Industry: Johnson & Johnson, Pfizer Inc., Merck & Co. Inc., AbbVie Inc., Novartis AG, Bayer AG, AstraZeneca plc, Corium International Inc., Alkermes plc, Amneal Pharmaceuticals
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=12587
Market Overview
Controlled release drug delivery systems are engineered to deliver medications in a controlled manner over extended periods. These systems include oral controlled release tablets, transdermal patches, injectable depots, implantable devices, and polymer-based carriers. In the U.S., such technologies are widely used in treating cardiovascular diseases, diabetes, cancer, neurological disorders, and autoimmune conditions.
The shift from immediate-release to controlled-release formulations is driven by the need to maintain consistent drug levels in the bloodstream, reduce peak-related side effects, and improve therapeutic efficiency. Additionally, pharmaceutical companies are increasingly adopting controlled release technologies to extend product lifecycles and differentiate branded drugs from generic competition.
Key Market Drivers
1. High Burden of Chronic Diseases
The U.S. has a large patient population suffering from chronic conditions.
Rising prevalence of diabetes, cardiovascular diseases, and cancer
Long-term medication requirements increase demand for sustained delivery
Need for improved treatment adherence
Controlled release systems help simplify complex treatment regimens.
2. Focus on Patient Compliance and Convenience
Medication non-adherence remains a major challenge in the U.S. healthcare system.
Reduced dosing frequency improves compliance
Once-daily or long-acting formulations enhance patient convenience
Lower risk of missed doses and medication errors
This makes controlled release therapies highly attractive to both patients and providers.
3. Technological Advancements in Drug Delivery
Continuous innovation is driving adoption across multiple therapeutic areas.
Polymer-based and biodegradable carriers
Nanotechnology-enabled controlled release systems
Advanced oral and injectable delivery platforms
These advancements enable more precise drug targeting and sustained release profiles.
4. Growth in Specialty and Biologic Drugs
The rise of complex biologics and specialty pharmaceuticals is boosting demand.
Controlled delivery improves stability of sensitive molecules
Long-acting injectables support biologic therapies
Reduced administration frequency enhances patient outcomes
Market Segmentation Analysis
By Delivery Technology
Oral Controlled Release Systems:
Widely used due to patient familiarity and ease of administration.
Injectable Controlled Release Formulations:
Preferred for long-acting therapies in oncology and chronic conditions.
Transdermal Drug Delivery:
Offers steady drug absorption and improved patient comfort.
Implantable Delivery Systems:
Used for highly targeted and long-duration therapies.
By Application Area
Cardiovascular Diseases:
Controlled release drugs help maintain stable blood pressure and heart function.
Oncology:
Sustained drug release improves efficacy while reducing toxicity.
Pain Management:
Long-acting formulations reduce the need for frequent dosing.
Neurological Disorders:
Controlled release supports consistent symptom control.
By End User
Pharmaceutical and Biotechnology Companies:
Primary developers and commercializers of controlled release drugs.
Hospitals and Clinics:
Major users, particularly for injectable and implantable systems.
Research Institutions:
Focused on innovation and next-generation delivery platforms.
Regional Demand Trends in the USA
Urban and Metropolitan Areas:
Higher adoption due to advanced healthcare infrastructure.
Academic Medical Centers:
Strong use in clinical trials and specialty treatments.
Outpatient Care Settings:
Growing demand for long-acting therapies that reduce hospital visits.
Challenges and Market Restraints
1. High Development and Manufacturing Costs
Complex formulation processes
Specialized equipment and expertise required
2. Regulatory and Approval Challenges
Stringent regulatory requirements for safety and efficacy
Extended clinical trial timelines
3. Technical Complexity
Ensuring consistent release profiles
Stability issues with certain drug molecules
Opportunities and Strategic Trends
1. Expansion of Long-Acting Injectable Therapies
Growing preference for monthly or quarterly dosing schedules.
2. Integration of Nanotechnology and Smart Polymers
Improves targeting precision and release control.
3. Lifecycle Management for Branded Drugs
Controlled release formulations help extend patent life and market exclusivity.
4. Growth in Home-Based and Outpatient Care
Long-acting drugs reduce hospital dependency and healthcare costs.
Future Outlook
The U.S. controlled release drug delivery market is expected to experience strong growth through 2035, driven by increasing chronic disease burden, technological advancements, and demand for patient-friendly therapies. As healthcare shifts toward value-based care, controlled release systems offer clear advantages by improving outcomes, reducing side effects, and enhancing adherence.
Pharmaceutical companies investing in advanced delivery technologies, personalized medicine, and long-acting formulations are likely to gain a competitive edge. Overall, controlled release drug delivery will remain a cornerstone of pharmaceutical innovation and patient-centric healthcare in the United States over the next decade.
Browse Full Report: https://www.factmr.com/report/united-states-controlled-release-drug-delivery
Purchase Full Report for Detailed Insights
For access to full forecasts, regional break-outs, product- and application-level analysis, company share details, and emerging trend assessments, you can purchase the complete report: https://www.factmr.com/checkout/12587
Have specific requirements or need assistance on report pricing or have a limited budget? Please contact sales@factmr.com
Related Reports:
Demand for Scar Treatment in UK: https://www.factmr.com/report/united-kingdom-scar-treatment-market
Demand for Animal Healthcare Packaging in UK: https://www.factmr.com/report/united-kingdom-animal-healthcare-packaging-market
Demand for Animal Healthcare Packaging in USA: https://www.factmr.com/report/united-states-animal-healthcare-packaging-market
Demand for Collagen in USA: https://www.factmr.com/report/united-states-collagen-market
Contact:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
About Fact.MR:
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Executive Report: Future of the USA Controlled Release Drug Delivery Indystry- Key Drivers, Disruption Signals & Industry Scenarios here
News-ID: 4315279 • Views: …
More Releases from Fact.MR

Citrus Fiber Market is Expanding at a 5.7% of CAGR by 2034 | Fact.MR Report
The global Citrus Fiber Market is projected to experience substantial growth over the next decade, driven by rising demand for clean-label ingredients, functional food components, and sustainable fiber sources.
Market analysts estimate that the market, valued at approximately USD 350 million in 2025, is expected to reach around USD 720 million by 2035, expanding at a compound annual growth rate (CAGR) of about 7.5% during the forecast period.
Get Access of…
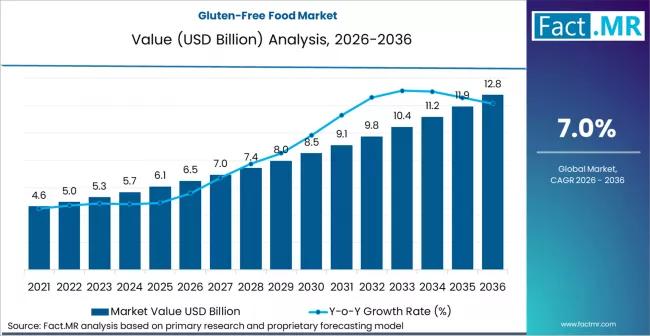
Gluten-Free Food Market is Predicted to Grow to USD 6.5 Billion in 2026 and USD …
The global gluten-free food market is entering a phase of mainstream consolidation, projected to grow from a valuation of USD 7.4 billion in 2026 to approximately USD 15.2 billion by 2036. This represents a steady compound annual growth rate (CAGR) of 7.5% over the ten-year forecast period.
While initially driven by medical necessity for celiac disease patients, the market is now being propelled by "lifestyle consumers" who perceive gluten-free products…
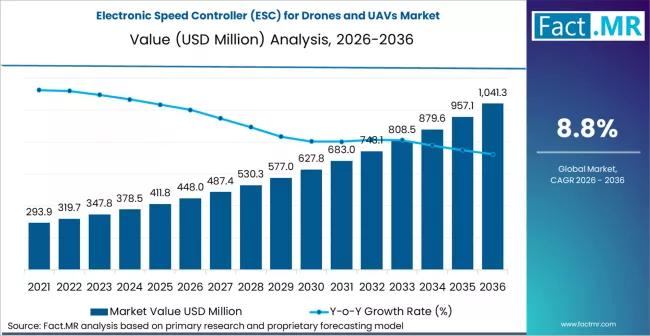
Electronic Speed Controller for Drones and UAVs Market is Valued USD 448.0 milli …
The global electronic speed controller (ESC) for drones and UAVs market is experiencing a rapid technological surge, projected to grow from a valuation of USD 1.8 billion in 2026 to approximately USD 5.1 billion by 2036. This represents a strong compound annual growth rate (CAGR) of 11.0% over the ten-year forecast period.
The market is being propelled by the proliferation of long-endurance commercial drones, the militarization of small FPV (First…
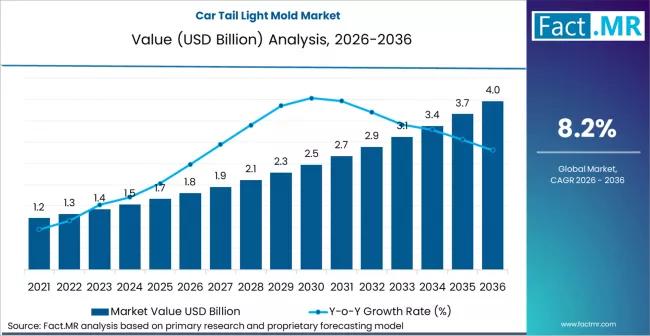
Car Tail Light Mold Market is Hoped-for USD 3.6 billion by 2036 | Fact.MR Report
The global car tail light mold market is navigating a high-design era, projected to grow from a valuation of USD 1.4 billion in 2026 to approximately USD 2.6 billion by 2036. This represents a compound annual growth rate (CAGR) of 6.4% over the forecast period.
The market is being fundamentally reshaped by the transition from simple bulbs to complex LED and OLED signatures, requiring high-precision multi-color and multi-material injection molding…
More Releases for Controlled
Automotive Silicon-Controlled Rectifier Market
Automotive Silicon-Controlled Rectifier Market Overview
Featuring immunity to surges and transients in static states and commutations, our silicon-controlled rectifiers (SCR), also known as thyristors, are ideal for single- and three-phase power line networks, even in harsh environments up to 150°C. In addition to AC, DC and capacitive ignitor circuits, these unidirectional switches are found in high-voltage industrial circuits as well as automotive equipment and all other segments where electromechanical relays are…
Passive Temperature Controlled Packaging Market 2023-2032: Temperature-Controlle …
In a world where the pharmaceutical industry is experiencing unprecedented growth due to an expanding global population, particularly the elderly, and a rising number of communicable and non-communicable diseases, the demand for both prescription and non-prescription drugs has never been higher. This surge in critical pharmaceutical needs has become a driving force behind the remarkable growth of the global passive temperature-controlled packaging market.
Allied Market Research has recently unveiled a…
Controlled Atmosphere Storage Using a DataTaker
DT80 Provides Single Solution & Saves Money
A company contacted CAS DataLoggers with a controlled atmosphere storage environment project as part of pilot testing for a new process. To reduce costs, existing equipment was used wherever possible. The hostile atmosphere produced was found to be incompatible with available humidity sensor technologies, requiring these measurements to be derived in real time using measurements from secondary sensors. The price of logging different sensors…
Temperature Controlled Packaging Systems Market
This report presents the worldwide Temperature Controlled Packaging Systems market size (value, production and consumption), splits the breakdown (data status 2013-2018 and forecast to 2025), by manufacturers, region, type and application. This study also analyzes the market status, market share, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, distributors and Porter’s Five Forces Analysis.
Download FREE Sample of this Report @ https://www.grandresearchstore.com/report-sample/global-temperature-controlled-packaging-systems-2025-971
The Temperature Controlled…
Controlled Drug Delivery Market Controlled Drug Delivery Clinical Pipeline Repor …
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1 Drug Delivery: An Advancing Trajectory
1.1 Preface Towards Drug Delivery
1.2 Idealism of Drug Delivery System
2. Introduction to Controlled Drug Delivery
2.1 Novel Drug Delivery Systems
2.2 Preamble to Controlled Drug Delivery
3. Evolution of Controlled Drug Delivery
3.1 First Generation
3.2 Second Generation
4. Fundamentals of Controlled Drug Delivery
4.1 Prerequisites…
Global Controlled Drug Delivery Market & Controlled Drug Delivery Clinical Trial …
With significant paradigm shifts in drug discovery and development, the industry is also gearing up to adopt alternative drug delivery strategies in order to further facilitate drug development. Opportunities have significantly increased in the last few years. Pharmaceutical companies are collaborating with drug delivery firms to effectively target patient concerns. Many different drug delivery systems have been designed and evaluated, and a large number of strategies for site-specific drug targeting…
