Press release
Endoscopic Vessel Harvesting Devices Market Growth in GCC and MENA: Top 5 Companies - KARL STORZ SE & Co. KG, Saphena Medical, Medical Instruments Spa, Sorin Group, Medical Instruments Spa
DataM Intelligence has published a new research report on "Endoscopic Vessel Harvesting Devices Market Size 2025". The report explores comprehensive and insightful Information about various key factors like Regional Growth, Segmentation, CAGR, Business Revenue Status of Top Key Players and Drivers. The purpose of this report is to provide a telescopic view of the current market size by value and volume, opportunities, and development status.Get Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/endoscopic-vessel-harvesting-devices-market?kb
United States: Recent Industry Developments
✅ November 2025: Medtronic launched an advanced endoscopic vessel harvesting system with enhanced imaging for minimally invasive cardiac surgeries.
✅ October 2025: Terumo Corporation expanded its device portfolio with new flexible harvesting tools to improve procedural efficiency.
✅ September 2025: FDA approved next-gen endoscopic vessel harvesting devices designed for faster recovery and reduced complications.
Japan: Recent Industry Developments
✅ November 2025: Olympus introduced high-definition imaging endoscopic harvesting devices for cardiovascular surgeries.
✅ October 2025: Nipro Corporation developed ergonomic harvesting tools tailored for minimally invasive procedures.
✅ September 2025: Collaboration between Japanese medical device firms and hospitals focused on training programs for advanced harvesting techniques.
GCC: Recent Industry Developments
✅ November 2025: Leading GCC hospitals adopted state-of-the-art endoscopic vessel harvesting devices to enhance cardiac surgery outcomes.
✅ October 2025: Regional distributors partnered with global manufacturers to improve device availability and technical support.
✅ September 2025: Healthcare authorities in the GCC launched initiatives to train surgeons on minimally invasive vessel harvesting technologies.
Key Players:
Getinge, Spectra Group, Terumo Corporation, KARL STORZ SE & Co. KG, Saphena Medical, Medical Instruments Spa, Sorin Group, Medical Instruments Spa, Med Europe S.r.l., and Olympus Corporation
Key Industry Development:
1. Saphenous vein harvesting for CABG remains the main indication, with disposable EVH systems accounting for about 65% of product share due to sterility, lower cross‐contamination risk, and simpler reprocessing.
2. Technology trends include improved endoscopic visualization, ergonomic handpieces, better smoke evacuation and regulated energy delivery, plus early moves toward robot‐assisted and AI‐guided EVH for more consistent dissection and fewer complications.
Key FDA and safety developments
1. On November 15, 2024, the FDA added EVH devices (product code GEI) to the medical device shortage list, reflecting constrained supply of certain systems and prompting risk‐mitigation guidance to providers.
2. The FDA announced a Class I recall and subsequent corrections for specific VasoView HemoPro EVH systems due to silicone separation and related safety concerns, leading to updated instructions for use issued in July 2025.
3. New device approval: Getinge Vasoview Hemopro 3
In March 2024, Getinge received FDA 510(k) clearance for its Vasoview Hemopro 3 EVH system, designed to enhance harvester efficiency and patient outcomes with improved smoke evacuation, optimized energy control, an ergonomic "game‐controller" handle and integrated cabling.
4. The system's U.S. launch was planned for Q3 2024 with registrations underway in additional key markets, positioning it as a next‐generation platform in the EVH space by 2025.
Clinical outcomes and practice patterns
1. Mid‐term outcome studies up to 2024-2025 report that EVH offers comparable graft patency and survival to open vein harvesting when performed by experienced teams, while reducing wound complications, pain, and length of stay.
2. Expert reviews in 2025 note that some earlier‐generation EVH systems are now withdrawn, and current practice is concentrating on a smaller set of modern platforms with stronger evidence, better ergonomics, and more robust training support.
Growth Forecast Projected:
The Global Endoscopic Vessel Harvesting Devices Market is anticipated to rise at a considerable rate during the forecast period, between 2025 and 2032. In 2024, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Research Process:
Both primary and secondary data sources have been used in the global Endoscopic Vessel Harvesting Devices Market research report. During the research process, a wide range of industry-affecting factors are examined, including governmental regulations, market conditions, competitive levels, historical data, market situation, technological advancements, upcoming developments, in related businesses, as well as market volatility, prospects, potential barriers, and challenges.
Buy Now & Get 30% OFF - Grab 50% OFF on 2+ reports: https://www.datamintelligence.com/buy-now-page?report=endoscopic-vessel-harvesting-devices-market?kb
Key Segments:
By Product Type: (EVH Systems, Accessories, Others)
By Application: (Coronary Artery Bypass Grafting (CABG), Peripheral Artery Bypass Surgery)
By End-user: (Hospitals, Ambulatory Surgical Centers (ASCs)
Regional Analysis for Endoscopic Vessel Harvesting Devices Market:
⇥ United States / North America: 38.6% market in 2024.
⇥ Europe: 30.3% market in 2024.
⇥ Asia‐Pacific: 20.5% market in 2024.
⇥ Japan: 15.2% market in 2024.
⇥ South Korea: 0.3% market in 2024.
Major EVH product launches and feature updates in 2025
1. Getinge Vasoview Hemopro 3 - launch ramp and positioning
2. Vasoview Hemopro 3 received FDA 510(k) clearance in March 2024, with U.S. launch beginning in Q3 2024 and wider market rollout through 2025.
3. Key features highlighted in 2024-2025 materials include enhanced smoke evacuation, regulated/balanced energy control to limit thermal spread, an ergonomic "game‐controller‐style" handle, and an integrated cable system to reduce clutter and setup time in the EVH field.
4. By mid‐2025, Getinge's interim report notes continued positive momentum in cardiovascular surgery, with Hemopro 3 cited among innovations supporting growth and ongoing regulatory interactions.
Terumo VirtuoSaph Plus and portfolio refresh
1. Terumo's VirtuoSaph Plus EVH system (an update of its long‐standing VirtuoSaph line) is promoted in 2025 as an endoscopic solution enabling single small incisions in the leg or arm, with emphasis on minimized scarring, reduced morbidity and infection risk, and improved patient recovery.
2. Marketing and technical descriptions stress refined visualization, improved ergonomics, and vessel preservation, positioning VirtuoSaph Plus as a premium option alongside Getinge's Vasoview series.
Technique/feature evolution highlighted by expert review (2025)
1. A 2025 expert panel review of EVH describes device‐level advances such as orientation rings at the endoscope tip for better spatial navigation, controlled CO2 insufflation to maintain a clear tunnel, and improved energy delivery and dissection tools-all reflecting incremental hardware and accessory refinements across leading platforms.
Get Customization in the report as per your requirements: https://datamintelligence.com/customize/endoscopic-vessel-harvesting-devices-market?kb
Regulatory actions or safety notices for EVH devices in 2025
Ongoing safety and shortage concerns (carry‐over from late 2024)
1. In November 2024, Getinge/Maquet issued an Urgent Medical Device Removal for VasoView HemoPro 1.5 (VH‐3500) and VasoView HemoPro 1 (VH‐3000‐W, OUS) after reports of silicone components detaching from the jaws; by January 2025, 17 serious injuries had been reported, prompting FDA to recommend immediate return of affected devices.
2. These issues led the FDA to add EVH systems (product code GEI) to the medical‐device shortage list and triggered ongoing scrutiny into other VasoView HemoPro models.
FDA Class I recall and 2025 EVH system correction
1. A Class I device recall was issued for certain VasoView HemoPro EVH systems due to risks of silicone detaching and heater wires bending/detaching during use, with potential for embolization or surgical complications.
2. On July 14, 2025, the FDA posted an "Endoscopic Vessel Harvesting (EVH) System Correction" notice covering HemoPro EVH systems, announcing updated Instructions for Use (IFU) to address bent/detached heater wires and peeling/detaching silicone, and detailing mitigation steps for clinicians.
Updated IFU and risk‐mitigation guidance
1. The FDA communication clarifies device‐handling precautions, inspection steps before and after procedures, and actions if silicone separation or wire issues are suspected, while advising facilities on inventory management amid ongoing supply constraints.
2. Professional‐society summaries in early 2025 emphasize that EVH remains acceptable when performed with current‐generation systems following updated IFUs, while legacy, recalled devices must be removed from use.
Unlock Free 360° Market Intelligence with DataM Subscription Services: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Endoscopic Vessel Harvesting Devices Market Growth in GCC and MENA: Top 5 Companies - KARL STORZ SE & Co. KG, Saphena Medical, Medical Instruments Spa, Sorin Group, Medical Instruments Spa here
News-ID: 4314751 • Views: …
More Releases from DataM Intelligence 4 Market Research LLP
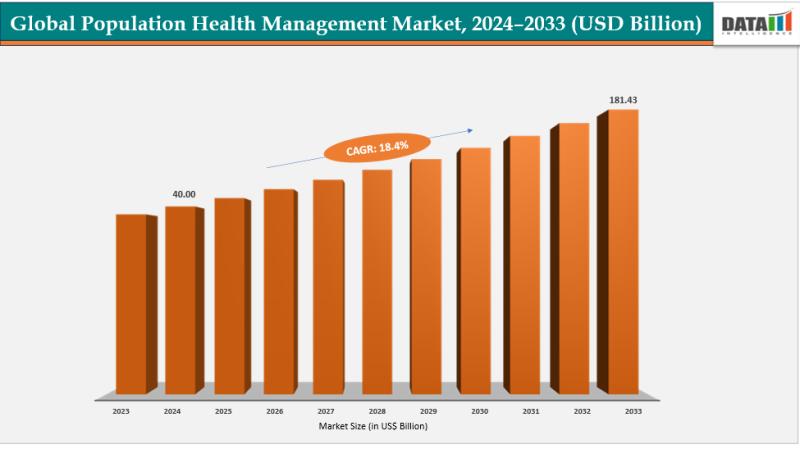
Population Health Management Market Set for Explosive Growth to USD 181.43 Billi …
The Global Population Health Management Market size reached USD 40.00 billion in 2024 and is expected to reach USD 181.43 billion by 2033, growing at a CAGR of 18.4% during the forecast period 2025-2033.
Market growth is driven by the rising prevalence of chronic diseases, increasing adoption of digital health solutions, and growing demand for value-based care models. Advancements in AI and predictive analytics, expanding healthcare IT infrastructure, surging investments in…

Organic Infant Formula Market Set to Grow to US$ 36,046 Million by 2032 at 6.3% …
AUSTIN, Texas and TOKYO -- According to DataM Intelligence, The Organic Infant Formula Market Size reached US$ 20,800 million in 2023, rose to US$ 22,110 million in 2024 and is projected to reach US$ 36,046 million by 2032, expanding at a CAGR of 6.3% from 2025 to 2032. The Organic Infant Formula Market is transforming early childhood nutrition by providing parents with certified organic, high-quality alternatives free from synthetic pesticides,…
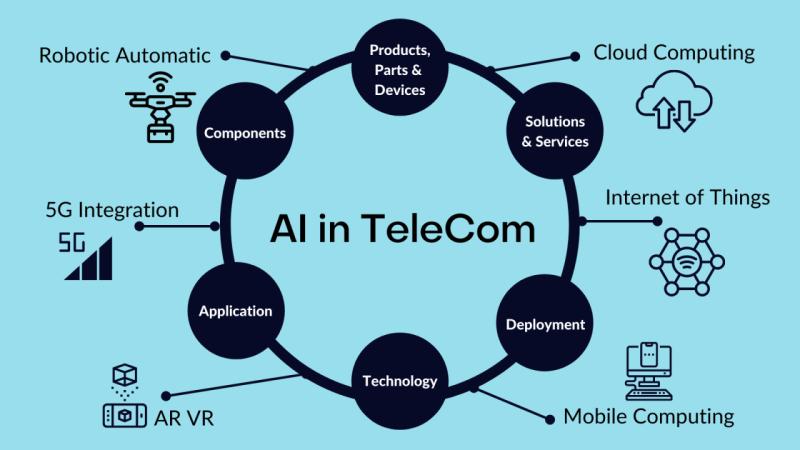
Future of Ai in telecommunication market. AI + Telecommunications Top Technologi …
The global AI in telecommunication market reached US$ 2.25 billion in 2023, with a rise to US$ 2.90 billion in 2024, and is expected to reach US$ 48.98 billion by 2033, growing at a CAGR of 36.9% during the forecast period 2025-2033.
AI in telecommunication market growth is driven by rising data traffic, demand for automated network optimization, predictive maintenance, improved customer experience, cost reduction, and rapid deployment of 5G and…

Bioresorbable Implants Market to Double, Reaching US$ 14.34 Billion by 2033 at 7 …
AUSTIN, Texas and TOKYO -- According to DataM Intelligence, The Bioresorbable Implants Market Size reached US$ 7.00 billion in 2024 and is projected to reach US$ 14.34 billion by 2033, expanding at a CAGR of 7.4% during the forecast period 2025-2033. The Bioresorbable Implants Market is transforming surgical outcomes by dissolving after fulfilling their role, leaving no permanent foreign body and lowering revision risks.
The shift from traditional metallic implants to…
More Releases for EVH
United States Endoscopic Vessel Harvesting Devices Market Outlook: Advancements …
The Endoscopic Vessel Harvesting Devices Market is valued at a significant CAGR during the forecast period (2024-2031).
Endoscopic Vessel Harvesting Devices are medical tools used in minimally invasive cardiac and vascular surgeries to carefully extract blood vessels, such as veins or arteries, from the body. These devices allow surgeons to harvest vessels through small incisions, reducing surgical trauma, minimizing scarring, and improving patient recovery compared to traditional open harvesting methods.
📌 Download…
Global Endoscopic Vessel Harvesting System Market Share 2021 Growth Factors, Ind …
Endoscopic vessel harvesting (EVH) is a surgical procedure, generally used to perform the minimally invasive procedures through very tiny incisions with an endoscope. It is an endoscopic approach in the saphenous vein harvesting. Using these systems, radial artery & saphenous vein can be removed from the leg for use as a bypass graft in the coronary surgery. The system’s endoscopic approach improves patient comfort and postoperative recovery after surgery. EVH…
Update in Lawsuit for Investors in shares of Evolent Health, Inc. (NYSE: EVH)
An investor, who purchased shares of Evolent Health, Inc. (NYSE: EVH), filed a lawsuit in August 2019 over alleged violations of Federal Securities Laws by Evolent Health, Inc..
Investors who purchased shares of Evolent Health, Inc. (NYSE: EVH) have certain options and for certain investors are short and strict deadlines running. Deadline: October 7, 2019. NYSE: EVH investors should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 -…
Deadline in Lawsuit for Investors in shares of Evolent Health, Inc. (NYSE: EVH) …
A deadline is coming up on October 7, 2019 in the lawsuit filed for certain investors of Evolent Health, Inc. (NYSE: EVH over alleged securities laws violations by Evolent Health, Inc.
Investors who purchased shares of Evolent Health, Inc. (NYSE: EVH) have certain options and there are strict and short deadlines running. Deadline: October 7, 2019. NYSE: EVH stockholders should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 -…
Lawsuit filed for Investors in shares of Evolent Health, Inc. (NYSE: EVH) I
An investor, who purchased shares of Evolent Health, Inc. (NYSE: EVH), filed a lawsuit over alleged violations of Federal Securities Laws by Evolent Health, Inc.
Investors who purchased shares of Evolent Health, Inc. (NYSE: EVH) have certain options and for certain investors are short and strict deadlines running. Deadline: October 7, 2019. NYSE: EVH investors should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779 - 1554.
Arlington, VA based Evolent…
Saphena Medical, Inc. Awarded Premier Purchasing Agreement for Venapax® Endosco …
On June 1, 2018 Saphena Medical Inc. was awarded a group purchasing agreement as a Breakthrough Technology with Premier, a national healthcare improvement company. Saphena Medical’s Venapax® Endoscopic Vessel Harvesting System is the first unitary EVH system on the market that allows for the saphenous vein or radial artery to be harvested for a coronary artery bypass graft procedure in a single comprehensive system. This agreement gives Premier members access…