Press release
Asia-Pacific Biosimilars Market Growth Accelerates with Rising Adoption & Manufacturing Expansion
Biosimilars Market | From Alternative Drugs to Global Health UtilityBiosimilars were once treated as lower-cost replicas of high-value biologics.
Today, they form the economic backbone of global therapeutic access.
Their role extends far beyond price. Biosimilars:
Shape treatment availability for cancer, autoimmune, and metabolic diseases
Influence hospital purchasing behavior
Support value-based healthcare models
Enable wider adoption of biologics in developing markets
Accelerate innovation cycles among key pharma players
This is why the global Biosimilars Market, valued at USD 31.6 billion in 2025, is on track to reach USD 125.9 billion by 2035, expanding at a powerful 13.4% CAGR.
This transformation will define the affordability and accessibility of healthcare for the next decade.
Get the Detailed Industry Analysis (including the Table of Contents, List of Figures, and List of Tables) - from the Biosimilars Market Research Report: https://marketgenics.co/reports/biosimilars-market-04169
Why Biosimilars Are Reshaping the Future of Global Healthcare
The reason is straightforward:
Chronic diseases dominate global health burden. Biologics dominate chronic disease treatment. Biosimilars dominate biologic affordability.
In oncology, rheumatology, endocrinology, nephrology, ophthalmology, and rare diseases, biosimilars are rapidly becoming the default treatment option.
Three structural forces shape this accelerating shift:
Patent Expiries Driving Open Competition
Dozens of blockbuster biologics-Humira, Herceptin, Remicade, Stelara, and more-are losing exclusivity.
Every expiry creates a multi-billion-dollar gap that biosimilars fill with:
30-50% lower treatment cost
Regulatory-validated clinical similarity
Fast-track approvals in mature markets
This transition is rewriting global healthcare economics.
Escalating Cost Pressure Across Health Systems
Governments, insurers, and hospital networks face unsustainable biologic expenditure.
Biosimilars convert:
high-cost therapies → affordable pathways
limited access → mass therapeutic reach
constrained delivery → scalable care
For example, hospitals increasingly prefer biosimilar trastuzumab, adalimumab, and rituximab, enabling oncology departments to treat more patients within the same budget.
Regulatory Support Accelerating Market Maturity
The EMA, FDA, PMDA (Japan), NMPA (China), CDSCO (India), and TGA (Australia) are strengthening:
interchangeability pathways
reference biologic transparency
manufacturing guidelines
accelerated approval schemes
Familiarity breeds adoption. Adoption breeds scale.
Scale accelerates the global biosimilars revolution.
To know more about the Biosimilars Market - Download our Sample Report: https://marketgenics.co/download-report-sample/biosimilars-market-04169
APAC | The Fastest-Emerging Powerhouse of the Biosimilars Market
While North America and Europe paved the early regulatory pathway, APAC is becoming the world's highest-potential growth engine in biosimilars.
Reasons APAC stands out:
Massive Chronic Disease Population
Asia hosts the world's largest burden of patients requiring long-term biologic therapy-oncology, diabetes, autoimmune diseases, and hormonal disorders.
Cost Sensitivity Drives Rapid Biosimilar Adoption
APAC health systems rely heavily on biosimilars to close affordability gaps.
Manufacturing Leadership
APAC is home to some of the world's most advanced biosimilar production ecosystems led by:
Biocon Biologics (India)
Samsung Bioepis (South Korea)
Celltrion (South Korea)
Shanghai Henlius (China)
Alvotech (operations in APAC)
Regulatory Evolution
China, India, Japan, South Korea, and Australia are accelerating biosimilar approvals and reshaping the competitive landscape.
APAC Will Deliver the Largest Incremental Market Opportunity by 2035
APAC is no longer catching up-
it is setting the pace for global biosimilar penetration and manufacturing scale.
Where the Biosimilars Market Gains Strategic Strength
Monoclonal Antibodies (mAbs) - The 36% Power Segment
mAbs dominate the market because they dominate disease burden:
Cancer
Rheumatoid arthritis
Multiple sclerosis
Autoimmune inflammatory conditions
With blockbuster mAbs losing exclusivity fast, biosimilar versions of:
Adalimumab
Trastuzumab
Rituximab
Bevacizumab
are becoming the treatment workhorses across hospitals, specialty clinics, and oncology centers.
Five Strategic APAC Biosimilar Hubs | Where Growth Accelerates
India | The Cost-Disruption Epicenter
India is emerging as a global manufacturing and R&D hub with companies like:
Biocon Biologics
Dr. Reddy's Laboratories
Intas Pharmaceuticals
Reliance Life Sciences
Strong regulatory momentum + cost-efficient production makes India a global supplier of complex biosimilars.
China | The Volume Giant
China's health system increasingly adopts biosimilars under:
cost-containment reforms
oncology initiative programs
expanded national reimbursement lists
Key Player Highlights: Henlius, Innovent, Bio-Thera Solutions
China's domestic scale makes it one of the most influential markets globally.
South Korea | The Innovation Vanguard
South Korea created one of the world's most sophisticated biosimilar ecosystems.
Key Players:
Samsung Bioepis
Celltrion
These companies are global leaders in manufacturing excellence, clinical trials, and advanced biologics.
Japan | High-Quality Adoption Market
Japan's cautious but strong regulatory clarity has accelerated adoption in:
oncology
rheumatology
endocrinology
With a rapidly aging population, biologic reliance is rising fast-making biosimilars essential.
Australia & Southeast Asia | The Fast-Adoption Corridor
Countries like Australia, Malaysia, Thailand, and Vietnam show:
rapid hospital-level adoption
strong oncology pipeline integration
favorable pricing and tender systems
APAC's diversity becomes its strength-different speeds, one direction.
Buy Now: https://marketgenics.co/buy/biosimilars-market-04169
Biosimilars Market Dynamics | The Forces Reshaping Global Therapeutics
Driver | Technology Advancements in Biologic Manufacturing
High-precision analytics, AI-supported similarity testing, and cell-line engineering reduce development risk and accelerate approvals.
Restraint | Regulatory Complexity & Interchangeability Challenges
Biosimilars require:
large-scale clinical validation
complex manufacturing
long approval cycles
Interchangeability still varies across markets.
Opportunity | Personalized, Affordable, Long-Term Therapies
The future lies in:
oncology & autoimmune personalized dosing
AI-driven biosimilar response prediction
real-world data platforms
This is where companies like Samsung Bioepis and Pfizer are already innovating.
The Biosimilars Market Is Becoming a Healthcare Utility Market
Future biosimilar leaders will not simply manufacture drugs.
They will manage:
real-world evidence ecosystems
AI-based treatment optimization
patient monitoring analytics
biologic lifecycle intelligence
digital medication adherence platforms
Biosimilars become not just therapies-
but data-driven health utilities.
This shifts competitive power from originator biologics to next-generation biosimilar platforms.
Get the complete market breakdown - statistics, insights, and future outlook: https://marketgenics.co/press-releases/biosimilars-market-04169
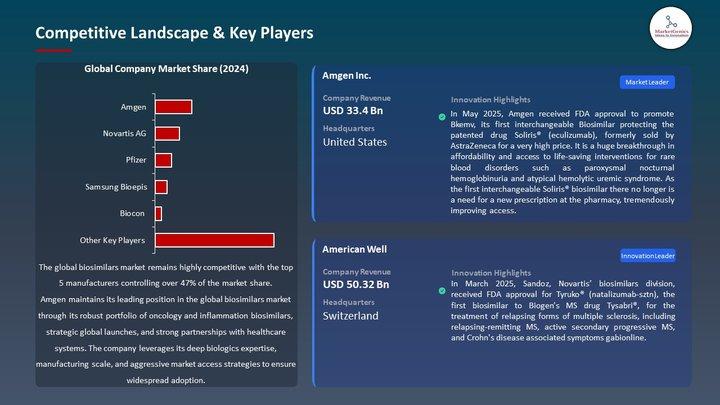
Key Players Transforming the Biosimilars Market
Global Leaders:
Amgen
Novartis/Sandoz
Pfizer
Roche
Samsung Bioepis
Biocon Biologics
Celltrion
Teva Pharmaceutical
Fresenius Kabi
Dr. Reddy's Laboratories
Alvotech
STADA
Xbrane
Merck KGaA
Boehringer Ingelheim
Shanghai Henlius
Coherus BioSciences
Mylan (Viatris)
Eli Lilly
Others
APAC players-Biocon, Celltrion, Samsung Bioepis, Intas, Dr. Reddy's-are rapidly becoming global forces.
Why This Biosimilars Market Report Matters for Pharma Leaders & Investors
Decision-makers don't need aspirational commentary.
They need actionable intelligence:
✓ Which therapies deliver the highest biosimilar conversion potential
✓ Where APAC presents the fastest scale-up opportunities
✓ How AI-driven monitoring platforms change provider adoption
✓ Which mAbs and biologic classes will generate the next $50B in biosimilar value
✓ How regulatory harmonization accelerates cross-border launches
✓ What competitive strategies will define 2035 market leadership
The Biosimilars Market 2025-2035 Report delivers this clarity with:
Real-world cost-outcome modeling
Regional adoption economics
Patent-to-entry timelines
Competitive intelligence
Technology impact forecasts
Own the Transition Before 2035 Defines It
Healthcare's future will not be limited by innovation-but by affordability.
Biosimilars convert:
biologic cost pressure → economic resilience
patient access gaps → universal therapeutic reach
complex treatment journeys → scalable, data-supported care
Those who treat biosimilars as strategic healthcare infrastructure will lead the next global healthcare cycle.
Those who treat them as cost alternatives will get left behind.
The next decade belongs to bold innovators, bold markets, and bold biosimilar ecosystems-especially across APAC.
Contact:
Mr. Debashish Roy
MarketGenics Research
800 N King Street, Suite 304 #4208, Wilmington, DE 19801, United States
USA: +1 (302) 303-2617
Email: sales@marketgenics.co
Website: https://marketgenics.co
About Us
MarketGenics is a global market research and management consulting company empowering decision makers across healthcare, technology, and policy domains. Our mission is to deliver granular market intelligence combined with strategic foresight to accelerate sustainable growth.
We support clients across strategy development, product innovation, healthcare infrastructure, and digital transformation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Asia-Pacific Biosimilars Market Growth Accelerates with Rising Adoption & Manufacturing Expansion here
News-ID: 4310609 • Views: …
More Releases from MarketGenics Research
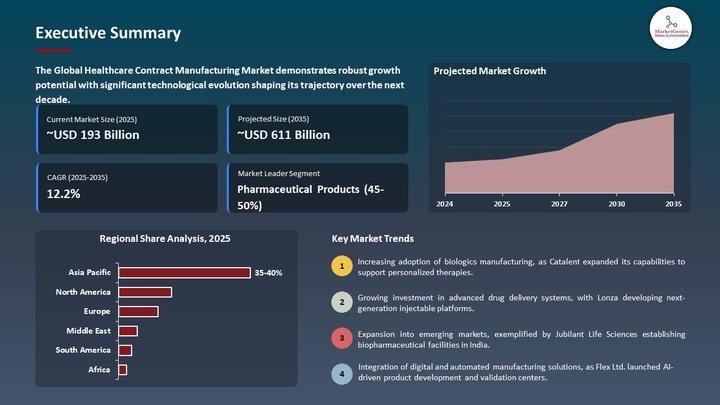
Healthcare Contract Manufacturing Market | Europe's Race for Quality-Centric Man …
Healthcare Contract Manufacturing Market | Europe's High-Precision Manufacturing Pivot Is Reshaping the Future of Therapeutics
The Healthcare Contract Manufacturing Market used to live in the operational shadows - a technical appendix to pharma strategy, an afterthought to medical device roadmaps.
That era is gone.
Europe's push for biologics scale-up, GMP modernization, sterile manufacturing compliance, and resilient supply chains has moved the Healthcare Contract Manufacturing Market from the backroom of operations into the center…

"Aerosol Cans Market in Europe: Sustainability, Aluminum Demand, and Regional Gr …
The world is moving fast on sustainability-biodegradable materials, reusable packaging, and recyclable metals are capturing headlines. Aerosol cans, often overlooked as simple packaging, have quietly evolved into a high-performance, environmentally-conscious solution across personal care, household, healthcare, and industrial sectors.
In 2025, the global Aerosol Cans Market reached USD 14.4 billion, and it is projected to expand to USD 24.0 billion by 2035, growing at a CAGR of 4.7%. For a sector…
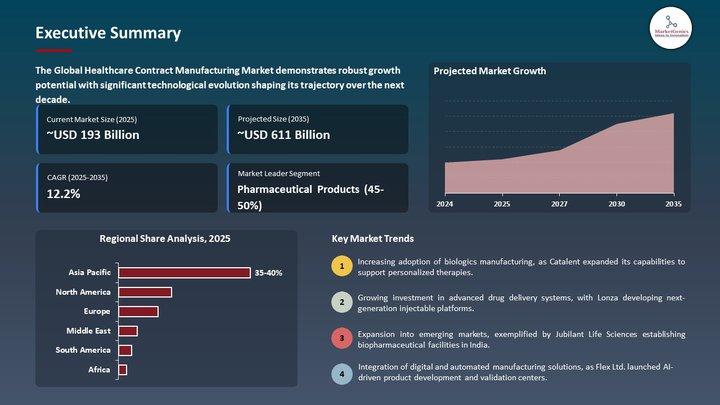
Clinical Trial Supplies Market | Europe's New Era of Trial Logistics - Big Pharm …
Clinical Trial Supplies Market | Europe's Supply-Chain Transformation Reshaping the Future of Drug Development
The Clinical Trial Supplies Market used to live in the back rooms of pharma operations - cartons, kits, comparators, storage rooms and shipping labels.
That era is gone.
Europe's pivot toward precision medicine, biologics, decentralized studies, and multi-country regulatory complexity has pushed the Clinical Trial Supplies Market from a logistics afterthought into a strategic pillar of clinical success.
This shift…
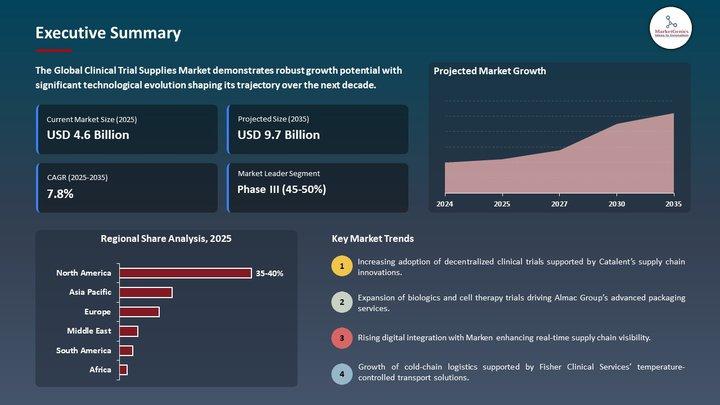
Clinical Trial Supplies Market | Europe's Supply-Chain Reinvention - Cold-Chain …
The Clinical Trial Supplies Market used to be a logistics afterthought: labelled vials, dry ice shipments, and predictable pallet runs. That era is gone.
Europe's regulatory complexity, the explosion of biologics and cell & gene therapies, and the rise of decentralized clinical trials (DCTs) have moved the Clinical Trial Supplies Market from a vendor line-item into a strategic capability that determines trial speed, quality and cost. From cryogenic storage in Frankfurt…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…