Press release
Top Companies in Thrombectomy Devices Market - Benchmarking Performance & Future Value Creation
The thrombectomy devices market is entering a pivotal phase as healthcare systems worldwide prioritize faster, more effective interventions for acute ischemic stroke, peripheral artery disease, and deep vein thrombosis. With rising global awareness of time-critical vascular emergencies and the need for minimally invasive procedures, thrombectomy devices are transitioning from specialized clinical tools to core technologies in emergency and interventional care. This shift is driven by rapid innovation in aspiration systems, stent retrievers, and mechanical thrombectomy platforms-supported by strong clinical evidence and increasing physician adoption.As hospitals expand stroke centers, adopt advanced imaging systems, and integrate AI-based triage solutions, leading manufacturers and emerging med-tech innovators are positioning themselves to capture growing clinical demand. The competitive environment is shaped by continuous R&D, strategic acquisitions, and the integration of robotics and real-time imaging into thrombectomy workflows.
➤ Request Free Sample PDF Report @ https://www.researchnester.com/sample-request-8029
Top Companies & Their Strategies
The thrombectomy devices market is driven by a combination of established medical device leaders and agile innovators focused on next-generation clot retrieval technologies. Their strategies reflect advancements in catheter design, physician training, clinical trials, and geographic expansion.
1. Stryker Corporation - Stryker is a dominant force in the neurovascular thrombectomy space, supported by its Trevo Retriever platform and broad neuro-interventional solutions portfolio. Its strategy focuses on continuous product refinement, physician education, and investment in comprehensive stroke center networks. Stryker's global footprint-especially in North America and Europe-enables large-scale distribution and strong clinical adoption.
2. Medtronic plc - Medtronic leverages its Solitaire X stent retriever system and robust R&D capabilities to maintain leadership in the thrombectomy devices market. The company's approach emphasizes clinical validation, partnerships with leading neurology institutes, and integration of imaging and robotic-assisted technologies. Medtronic's large installed customer base and diversified product ecosystem offer a substantial competitive advantage.
3. Penumbra, Inc. - Penumbra has built its leadership through aspiration-based thrombectomy devices, such as the Penumbra Indigo and Penumbra System. The company's strategy revolves around expanding its aspiration technology into broader vascular applications, including deep vein thrombosis and pulmonary embolism. Penumbra's specialization, innovation pace, and strong relationships with interventional radiologists fuel its market growth.
4. Boston Scientific Corporation - Boston Scientific focuses on mechanical thrombectomy for peripheral vascular disease, supported by a deep pipeline and strong acquisitions. The company's strategy includes expanding minimally invasive treatment options, enhancing physician training programs, and deepening its reach in emerging markets. Boston Scientific's strong financial stability allows for continuous investment in new technologies and strategic collaborations.
➤ Explore detailed profiles of top players and new entrants in this space - access your free sample report → https://www.researchnester.com/sample-request-8029
5. Johnson & Johnson (Cerenovus) - Cerenovus, a subsidiary of Johnson & Johnson, develops advanced neurovascular thrombectomy devices including the EMBOTRAP Revascularization Device. The company's strategy emphasizes evidence-based design, clinical trials, and next-generation device engineering. Its access to J&J's global network enhances commercialization capacity and supports long-term research initiatives.
6. Terumo Corporation - Terumo is expanding rapidly with innovative aspiration and catheter-based thrombectomy systems designed for both neurovascular and peripheral interventions. The company's strategy focuses on product differentiation through advanced microcatheter engineering, strong distribution networks across Asia-Pacific, and continuous clinician engagement. Terumo's cost-efficient production model offers a competitive advantage in emerging regions.
7. Abbott Laboratories - Abbott leverages its expertise in vascular interventions to penetrate the thrombectomy devices market with systems built for peripheral artery disease. Its strategy includes expanding indications, integrating advanced imaging support tools, and strengthening collaborations with interventional cardiologists. Abbott's global operational scale and strong brand trust enhance its competitive stance.
8. Inari Medical - Inari is a rising disruptor specializing in large-vessel thrombectomy for venous thromboembolism (VTE). Its ClotTriever and FlowTriever systems offer mechanical solutions without the need for thrombolytics, positioning the company uniquely in the market. Inari's rapid innovation cycle, strong clinical outcomes, and targeted commercialization strategy have earned it significant attention from investors and clinicians.
➤ View our Thrombectomy Devices Market Report Overview here: https://www.researchnester.com/reports/thrombectomy-devices-market/8029
SWOT Analysis
Strengths - Leading companies benefit from strong R&D pipelines, extensive clinical validation, and established relationships with neuro and vascular intervention specialists. Their diverse product portfolios-ranging from aspiration systems to stent retrievers-enable comprehensive treatment options. Innovation in catheter design, device navigability, and clot retrieval efficiency strengthens their competitive positioning. Global distribution networks and physician training platforms further support rapid clinical adoption.
Weaknesses - High device costs and the need for specialized clinical expertise pose challenges for widespread adoption, particularly in emerging markets. Companies also face operational complexity due to stringent regulatory requirements and the need for continuous post-market surveillance. The dependence on hospital infrastructure such as advanced imaging equipment can limit market penetration. Additionally, device malfunctions or clinical failures carry significant reputational risk.
Opportunities - Increasing global incidence of stroke and vascular diseases creates expanding clinical demand for thrombectomy devices. Growth in dedicated stroke centers, tele-stroke programs, and AI-based triage tools enhances the feasibility of rapid interventions. Emerging markets across Asia-Pacific and Latin America are investing heavily in advanced interventional care. Newer areas such as pulmonary embolism thrombus removal, venous thrombectomy, and robotic-assisted navigation offer substantial untapped potential.
Threats - Growing competition among established and emerging device manufacturers may lead to pricing pressure and reduced margins. Supply chain disruptions affecting specialized materials-such as microcatheters and nitinol-pose operational risks. Evolving clinical guidelines and stringent regulatory pathways can delay product launches. Alternative treatments, including pharmacological thrombolysis or hybrid surgical approaches, could reduce demand in certain segments.
➤ Access a complete SWOT breakdown with company-specific scorecards: Claim your sample report → https://www.researchnester.com/sample-request-8029
Investment Opportunities & Trends
The thrombectomy devices market is witnessing robust investment activity as healthcare systems prioritize improved outcomes for stroke and vascular emergencies. Investors are focusing on companies advancing device precision, expanding indications, and integrating digital and robotic technologies.
1. Mergers & Acquisitions (M&A)
Major medical device companies have pursued acquisitions to strengthen their neurovascular and peripheral intervention portfolios. Industry consolidation has been driven by the need for advanced catheter technologies, next-generation aspiration devices, and AI-enabled imaging support. Acquisitions targeting device startups with proprietary designs or technology patents remain a major investment theme.
2. Funding in Startups
Venture capital funding has increased for startups focused on mechanical and aspiration-based thrombectomy. Early-stage innovators working on ultra-low profile catheters, robotic navigation, and clot characterization systems have attracted substantial investment. Startups specializing in venous thrombectomy and pulmonary embolism management are gaining particular interest due to the expanding treatment landscape.
3. Technology Integration
AI-powered imaging, automated stroke detection platforms, and robotics are becoming central differentiators for device manufacturers. Technology integration supports faster decision-making, enhances procedural accuracy, and expands treatment windows. Companies are investing in advanced materials to improve flexibility, clot engagement, and safety profiles.
4. Regional Expansion
North America remains the leading hub for thrombectomy innovation, supported by strong clinical trial infrastructure and funding. Europe is accelerating adoption with new stroke network setups and supportive clinical guidelines. Asia-Pacific, particularly China, Japan, and India, is emerging as a high-growth region due to expanding hospital capabilities and government investments in stroke care systems.
Notable Developments in the Past 12 Months
• Multiple companies launched next-generation aspiration catheters and stent retrievers with enhanced clot removal efficiency.
• Emerging startups secured new funding rounds to develop robotic-assisted thrombectomy systems.
• Regulatory agencies adopted updated stroke treatment pathways promoting faster intervention windows.
• Large device manufacturers expanded strategic partnerships with neurovascular centers for training and clinical trials.
• Asia-Pacific hospitals saw accelerated adoption of thrombectomy technologies through public-private investments.
➤ Request Free Sample PDF Report @ https://www.researchnester.com/sample-request-8029
Contact Data
AJ Daniel
Corporate Sales, USA
Research Nester
77 Water Street 8th Floor, New York, 10005
Email: info@researchnester.com
USA Phone: +1 646 586 9123
Europe Phone: +44 203 608 5919
About Research Nester
Research Nester is a one-stop service provider with a client base in more than 50 countries, leading in strategic market research and consulting with an unbiased and unparalleled approach towards helping global industrial players, conglomerates and executives for their future investment while avoiding forthcoming uncertainties. With an out-of-the-box mindset to produce statistical and analytical market research reports, we provide strategic consulting so that our clients can make wise business decisions with clarity while strategizing and planning for their forthcoming needs and succeed in achieving their future endeavors. We believe every business can expand to its new horizon, provided a right guidance at a right time is available through strategic minds.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Top Companies in Thrombectomy Devices Market - Benchmarking Performance & Future Value Creation here
News-ID: 4309976 • Views: …
More Releases from Research Nester Pvt Ltd

Key Players in the Returnable Packaging Market: Share Positioning & Investor Per …
The returnable packaging market is gaining strategic importance as companies across logistics, food & beverage, automotive, and retail industries seek cost efficiency, sustainability, and supply chain resilience. Returnable packaging solutions-such as reusable pallets, crates, containers, drums, and intermediate bulk containers (IBCs)-are increasingly favored over single-use packaging due to regulatory pressure, circular economy goals, and operational efficiency.
➤ Request Free Sample PDF Report @ https://www.researchnester.com/sample-request-8352
Top Companies
1. Brambles (CHEP)
Brambles, through its…

Pressure Control Equipment Market Dominance: Top Companies Strengthening Share & …
Pressure control equipment plays a mission-critical role in ensuring safety, operational integrity, and regulatory compliance across oil & gas exploration, well intervention, drilling, and production activities. From blowout preventers (BOPs) and control heads to manifolds and pressure valves, these systems are essential for managing high-pressure environments in both onshore and offshore operations.
As upstream operators focus on deeper wells, high-pressure high-temperature (HPHT) environments, and complex well architectures, demand for advanced pressure…
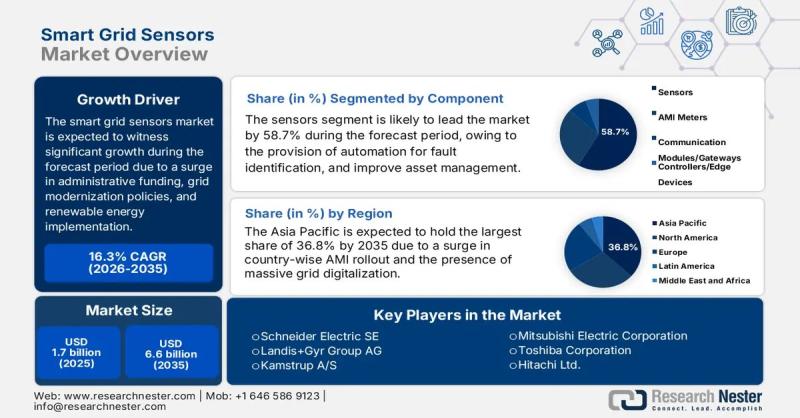
Smart Grid Sensors Market size to exceed $6.6 Billion by 2035 | General Electric …
Market Outlook and Forecast
The smart grid sensors market is emerging as a critical enabler of next-generation power infrastructure, supporting utilities and grid operators in transitioning from conventional, centralized electricity networks to intelligent, data-driven energy systems. Smart grid sensors provide real-time visibility into grid performance, enabling advanced monitoring, predictive maintenance, fault detection, and efficient energy distribution across transmission and distribution networks.
In 2025, the global smart grid sensors market is valued at…

Top Companies in Architectural Lighting Market - Benchmarking Performance & Futu …
The architectural lighting market has evolved into a design-driven, technology-intensive segment of the global lighting industry. Beyond illumination, architectural lighting now plays a critical role in enhancing aesthetics, supporting energy efficiency goals, and enabling smart building environments. Demand is increasingly shaped by urban development, commercial real estate upgrades, hospitality projects, and public infrastructure modernization.
➤ Request Free Sample PDF Report @ https://www.researchnester.com/sample-request-8342
Top Companies & Their Strategies
1. Signify (Philips Lighting)
Signify…
More Releases for Device
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Surge In Wireless Device Usage Boosts Wireless Audio Device Market Driving Marke …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Wireless Audio Device Market Size By 2025?
In recent years, there has been remarkable growth in the wireless audio device market size. The market, which is projected to expand from $41.85 billion in 2024 to $52.37 billion in 2025, boasts a compound annual growth rate (CAGR) of 25.1%. Factors contributing…
Anti-snoring Device Market - Quiet Nights, Restful Sleep: Anti-snoring Device In …
Newark, New Castle, USA: The "Anti-snoring Device Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Anti-snoring Device Market: https://www.growthplusreports.com/report/antisnoring-device-market/8931
This latest report researches the industry structure, sales, revenue,…
Global Watch Clock Measuring Device Market | Watch Clock Measuring Device Indust …
Watch, clock and measuring device market comprises of the sales of watch, clock, measuring device & related services to measure the time and physical quantity. Watch is portable timepiece, which is worn by people around the wrist, attached by a strap. Clock is a device used to measure and indicate time, using the pointers moving over a dial. Measuring device is an instrument used for measuring the various parameters in…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Medical Device Technologies Market - The Evolution of Medical Device Technologie …
The global medical device technologies market is anticipated to be boosted by various well-known players in the market. Some of these players that are dealing with the manufacturing of in vitro diagnostic devices hold a significant share in the global market. Whereas, the small market players are emerging from several developing nations, looking to set their foot in the market. Such measures are foreseen to change the market scenario in…