Press release
Clinical Research Organization Business Plan 2025 : Feasibility, Investment Needs, and Market Trends
Clinical Research Organization Business Plan & Project Report OverviewIMARC Group's "Clinical Research Organization Business Plan and Project Report 2025" offers a comprehensive framework for establishing a successful clinical research organization business. The critical areas, including market trends, investment opportunities, revenue models, and financial forecasts, are discussed in this in-depth report and are therefore useful resources to entrepreneurs, consultants and investors. Whether evaluating the viability of a new venture or streamlining an existing one, the report gives an in-depth analysis of all the ingredients that make it successful, starting with business formation and profitability over time.
What is a Clinical Research Organization Business?
A professional services organization that provides a thorough range of specialized, precise and scientific services dedicated to the management of clinical trials and to pharmaceutical research, usually with adaptive methodology, regulatory expertise, information technology services, data collection and management, patient recruitment, clinical monitoring and evidence generation in a manner specific to a given drug's indications. CROs expedite the development of clinical drugs for the pharmaceutical, biopharmaceutical, medical device and biomedical industries.
These services include protocol facilitation, clinical trial design and site selection and management, patient recruitment and retention, data collection and analysis, regulatory affairs submission, pharmacovigilance, biostatistics, medical writing, and any quality assurance programs within an organization that is involved in the development of therapeutics through protocol-driven clinical research.
Full-service and specialty CROs, site management organizations, and clinical data management organizations work under the same model, valuing scientific validity, regulatory compliance, protocol standards, investigator training, patient safety, data integrity, ethics committee coordination, and stakeholder collaboration throughout the entire life cycle of a clinical trial.
To this end, Clinical Research Organizations (CROs) rely on electronic data capture, clinical trial management systems, regulatory intelligence software, patient recruitment software, adverse event reporting software, statistical analysis software, document management software and research performance analytics software.
These sites may include global full-service CROs, therapeutic area specialists, early-phase research sites and full-development sites which provide end-to-end levels of research execution across various therapeutic areas, clinical trial phases and levels of regulatory complexity.
Request for a Sample Report: https://www.imarcgroup.com/clinical-research-organization-business-plan-project-report/requestsample
Clinical Research Organization Business Market Trends and Growth Drivers
Trends and drivers of growth: Demand in the Clinical Research Organization business is driven by growth in pharmaceutical R&D spending, increasing protocol complexity, increased need for specialized research services and need to reduce time to market drugs. In addition, the following trends are impacting the business: demand for precision medicine, shift towards protocol design that is patient-centric, decentralized and real-world evidence (RWE), increasing sponsors' preference to enter into risk-sharing agreements, therapeutic area focus and increasing access to patient groups in the evolving pharmaceutical development landscape.
To meet that increased demand, our customers are investing in the electronic data capture systems, global investigator databases, patient-recruitment systems, regulatory compliance and Good Clinical Practice (GCP) applications that improve not only the conduct of their research, but the performance of their business in an industry that faces increasing pressure to innovate and to improve regulatory efficiency.
Diversifying revenue sources is another way to increase financial resiliency. Besides clinical trial management services, these can include contracts for regulatory consulting, biostatistics, medical writing, data management, pharmacovigilance monitoring, investigator training, and feasibility assessment studies, which are billed separately from clinical trial management fees.
Location and network strength. Organizations situated in areas with strong investigator networks, patient populations and healthcare infrastructure as well as good access to research sites tend to enjoy favorable working conditions and competitive advantages. Simultaneously, cutting-edge data management systems and compliance with international regulatory and ethical research standards ensure operational excellence and the confidence of the sponsors.
Additional risks to consider include dynamic regulatory standards that impact the organization's standard operating procedures, competition from already established larger global CROs and emerging small research companies around the world, reliance on sponsor funding cycles and the state of the pharmaceutical industry, and regulatory standards for data integrity and patient safety requirements.
Establishing a successful Clinical Research Organization (CRO) business model requires large investments in technology infrastructure and secured data storage facilities, research-related software systems, and in quality management. It also requires the recruitment of well-educated and trained clinical research personnel, regulatory and quality specialists, project leaders, and data analysts and scientists. Along with business development work, in which deep business relationships and trust with sponsors is built, an important focus is on long-term partnering with pharmaceutical sponsors, biotechnology sponsors and medical device sponsors. By providing superior research delivery, data quality, and customer service, such companies can do medical science while helping sponsors bring new life-saving therapies to patients via efficient clinical development pathways.
Report Coverage
The Clinical Research Organization Business Plan and Project Report includes the following areas of focus:
• Business Model & Operations Plan
• Technical Feasibility
• Financial Feasibility
• Market Analysis
• Marketing & Sales Strategy
• Risk Assessment & Mitigation
• Licensing & Certification Requirements
The comprehensive nature of this report ensures that all aspects of the business are covered, from market trends and risk mitigation to regulatory requirements and client-focused business development strategies.
Key Elements of Clinical Research Organization Business Setup
Business Model & Operations Plan
A solid business model is crucial to a successful venture. The report covers:
• Service Overview: A breakdown of clinical trial management, protocol development, site selection and monitoring, patient recruitment services, data management and biostatistics, regulatory affairs consulting, pharmacovigilance monitoring, medical writing, and quality assurance services offered
• Service Workflow: How each study startup, site activation, patient enrollment, data collection, monitoring visit, adverse event reporting, database lock, and regulatory submission process is managed
• Revenue Model: An exploration of the mechanisms driving revenue across multiple therapeutic areas and research services
• SOPs & Service Standards: Guidelines for consistent research quality, data integrity standards, regulatory compliance practices, and client satisfaction
This section ensures that all operational and quality aspects are clearly defined, making it easier to scale and maintain service excellence.
Buy Report Now: https://www.imarcgroup.com/checkout?id=43343&method=1911
Technical Feasibility
Setting up a successful business requires proper research infrastructure planning. The report includes:
• Location Selection Criteria: Key factors to consider when choosing office locations and target research markets
• Space & Costs: Estimations for required office space, data management facilities, monitoring operations centers, and associated costs
• Equipment & Systems: Identifying essential clinical trial management software, electronic data capture platforms, document management systems, and regulatory intelligence technology
• Facility & Technology Setup: Guidelines for creating secure research operations facilities and compliant data management infrastructure
• Utility Requirements & Costs: Understanding the IT infrastructure and utilities necessary to run clinical research operations
• Human Resources & Wages: Estimating staffing needs, roles, and compensation for clinical research associates, project managers, data managers, biostatisticians, regulatory specialists, and support personnel
This section provides practical, actionable insights into the research infrastructure needed for setting up your business, ensuring regulatory compliance and operational excellence.
Financial Feasibility
The Clinical Research Organization Business Plan and Project Report provides a detailed analysis of the financial landscape, including:
• Capital Investments & Operating Costs: Breakdown of initial and ongoing investments
• Revenue & Expenditure Projections: Projected income and cost estimates for the first five years
• Profit & Loss Analysis: A clear picture of expected financial outcomes
• Taxation & Depreciation: Understanding tax obligations and technology depreciation
• ROI, NPV & Sensitivity Analysis: Comprehensive financial evaluations to assess profitability
This in-depth financial analysis supports effective decision-making and helps secure funding, making it an essential tool for evaluating the business's potential.
Market Insights & Strategy
Market Analysis
A deep dive into the clinical research organization market, including:
• Industry Trends & Segmentation: Identifying emerging trends and key market segments across full-service CROs, specialized therapeutic area providers, site management organizations, functional service providers, and laboratory services
• Regional Demand & Cost Structure: Regional variations in clinical research outsourcing and cost factors affecting CRO operations
• Competitive Landscape: An analysis of the competitive environment including established global CROs, regional research organizations, specialized niche providers, and academic research institutions
Profiles of Key Players
The report provides detailed profiles of leading players in the industry, offering a valuable benchmark for new businesses. It highlights their strategies, service offerings, therapeutic specializations, and market positioning, helping you identify strategic opportunities and areas for differentiation.
Capital & Operational Expenditure Breakdown
The report includes a comprehensive breakdown of both capital and operational costs, helping you plan for financial success. The detailed estimates for facility development, technology systems, and operating costs ensure you're well-prepared for both initial investments and ongoing expenses.
• Capital Expenditure (CapEx): Focused on office space setup and design, clinical trial management software, electronic data capture systems, data security infrastructure, document management platforms, and regulatory compliance technology
• Operational Expenditure (OpEx): Covers ongoing costs like staff salaries, technology licensing fees, investigator payments, travel expenses, regulatory submission costs, insurance premiums, and facility maintenance
Financial projections ensure you're prepared for cost fluctuations, including adjustments for technology upgrade cycles, competitive pricing pressures, regulatory requirement changes, and market demand variations over time.
Profitability Projections
The report outlines a detailed profitability analysis over the first five years of operations, including projections for:
• Total revenue from clinical trial management, regulatory consulting, data management services, and specialized research offerings, expenditure breakdown, gross profit, and net profit
• Profit margins for each service line and year of operation
• Revenue per study projections and market penetration growth estimates
These projections offer a clear picture of the expected financial performance and profitability of the business, allowing for better planning and informed decision-making.
Request For Customization: https://www.imarcgroup.com/request?type=report&id=43343&flag=E
Our expertise includes:
• Market Entry and Expansion Strategy
• Feasibility Studies and Business Planning
• Company Incorporation and Clinical Research Setup Support
• Regulatory and Licensing Navigation
• Competitive Analysis and Benchmarking
• Industry Partnership Development
• Branding, Marketing, and Client-Focused Business Strategy
About Us
IMARC Group is a leading global market research and management consulting firm. We specialize in helping organizations identify opportunities, mitigate risks, and create impactful business strategies.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: (+1-201971-6302)
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Research Organization Business Plan 2025 : Feasibility, Investment Needs, and Market Trends here
News-ID: 4289257 • Views: …
More Releases from IMARC Group
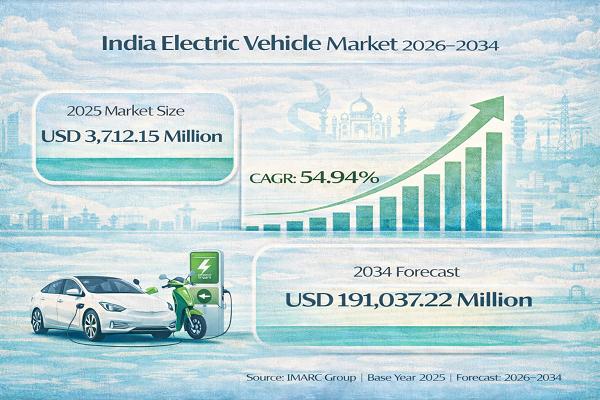
India Electric Vehicle Market Set to Reach USD 191,037.22 Million by 2034, Expan …
India Electric Vehicle Market : Report Introduction
According to IMARC Group's report titled "India Electric Vehicle Market Size, Share, Trends and Forecast by Vehicle Type, Price Category, Propulsion Type, and Region, 2026-2034" the report offers a comprehensive analysis of the industry, including market share, growth, trends, and regional insights.
Free Sample Download PDF (Exclusive Offer on Corporate Email) : https://www.imarcgroup.com/india-electric-vehicle-market/requestsample
India Electric Vehicle Market Overview
The India electric vehicle market size was valued at…
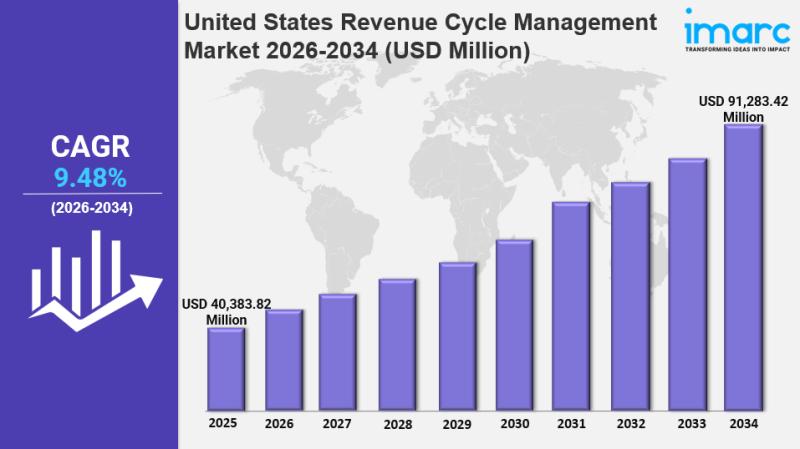
United States Revenue Cycle Management Market Size, Trends, Growth and Forecast …
IMARC Group has recently released a new research study titled "United States Revenue Cycle Management Market Size, Share, Trends and Forecast by Type, Component, Deployment, End User, and Region, 2026-2034", offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends and competitive landscape to understand the current and future market scenarios.
Connect with a Research Analyst Now:
https://www.imarcgroup.com/united-states-revenue-cycle-management-market/requestsample
United States Revenue Cycle Management Market Summary:
The United States revenue cycle…

LED Chip Manufacturing Plant Cost Report 2026: Demand Analysis, CapEx/OpEx & ROI …
Setting up an LED chip manufacturing plant involves strategic planning, substantial capital investment, and comprehensive understanding of semiconductor fabrication technologies. These high-performance components power everything from general illumination and displays to automotive lighting and consumer electronics. Success requires careful site selection, advanced epitaxial growth processes, sophisticated cleanroom facilities, reliable raw material sourcing, and compliance with stringent quality and environmental regulations to ensure profitable and sustainable operations.
IMARC Group's report, "LED Chip…

Eyewear Manufacturing Plant DPR & Unit Setup - 2026: Machinery Cost, CapEx/OpEx, …
Setting up an eyewear manufacturing plant positions investors within a strategically important segment of the global optical and fashion accessories industry, driven by increasing demand for vision correction solutions, rising awareness of eye health, and growing fashion consciousness. As modern lifestyles advance, digital device usage expands, and the need for protective and corrective eyewear grows, eyewear continues to gain traction across prescription glasses, sunglasses, safety eyewear, and fashion accessories worldwide.…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
