Press release
Anti-CD38 Antibody Clinical, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline Analysis | I-MAB Biopharma, Takeda, HLX15, Ancora Biotech, CASI Pharmaceuticals
DelveInsight's, "Anti-CD38 antibody - Pipeline Insight, 2025" report provides comprehensive insights about 10+ companies and 12+ pipeline drugs in Anti-CD38 antibody pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.According to DelveInsight, the Anti-CD38 antibody pipeline features over 10 leading companies actively engaged in developing more than 12 therapeutic candidates targeting CD38.
Anti-CD38 antibody Overview:
CD38 is a membrane-associated protein first identified through monoclonal antibody studies of lymphocytes, leading to its initial classification as a lymphocyte-specific marker. Discovered in 1980 by E.L. Reinherz and S. Schlossman, CD38 is a type II transmembrane glycoprotein that plays vital roles in cell migration, receptor-mediated adhesion (through interactions with CD31 or hyaluronic acid), and several intracellular signaling pathways. Its expression in lymphocytes varies by developmental stage, and activation with agonistic CD38 antibodies can trigger distinct cellular responses across different blood cell types. Beyond the cell surface, CD38 is also found within intracellular compartments, including the nucleus.
A pivotal finding was its structural resemblance to ADP-ribosyl cyclase, broadening the understanding of CD38's enzymatic functions. It is now recognized as a multifunctional enzyme involved in the metabolism of calcium-signaling messengers such as cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP). These molecules regulate calcium release-cADPR through ryanodine receptors in the endoplasmic reticulum and NAADP through calcium stores in lysosomes and other acidic organelles.
Structurally, CD38 is a 46 kDa type II transmembrane glycoprotein with a short cytoplasmic N-terminal tail and a large extracellular catalytic domain. It can be internalized, released, or shed as a soluble 39 kDa form present in body fluids. The CD38 gene, located on a human chromosome, is widely expressed across hematopoietic and non-hematopoietic tissues, with expression levels modulated by activation and differentiation signals. CD38 is found on activated T and B cells, monocytes, NK cells, dendritic cells, and plasma cells, as well as on regulatory immune cell populations such as Tregs, Bregs, and myeloid-derived suppressor cells. Elevated CD38 expression in these cells can contribute to immune suppression, particularly in cancer settings.
Request for a detailed insights report on Anti-CD38 antibody pipeline insights [https://www.delveinsight.com/report-store/anti-cd38-antibody-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
"Anti-CD38 antibody Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Anti-CD38 antibody Therapeutics Market.
Key Takeaways from the Anti-CD38 antibody Pipeline Report
*
DelveInsight's Anti-CD38 Antibody Pipeline Report highlights a dynamic and competitive landscape, with over 10 active companies advancing 12+ investigational therapies targeting CD38.
*
On July 2, 2025, the FDA granted accelerated approval for an Anti-CD38 antibody therapy indicated for adults with relapsed or refractory multiple myeloma who have received four or more prior treatments, including other anti-CD38 monoclonal antibodies.
*
Leading players in this space include I-MAB Biopharma, Takeda, HLX15, Ancora Biotech, CASI Pharmaceuticals, and several others, all striving to enhance treatment outcomes through novel mechanisms and optimized antibody engineering.
*
Notable Anti-CD38 antibody candidates under development include TAK-079, HLX15, CID-103, among others, progressing through various stages of clinical evaluation.
Anti-CD38 antibody Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Anti-CD38 antibody Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Anti-CD38 antibody treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Anti-CD38 antibody market.
Download our free sample page report on Anti-CD38 antibody pipeline insights [https://www.delveinsight.com/sample-request/anti-cd38-antibody-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Anti-CD38 antibody Emerging Drugs
TAK-079 - Takeda
TAK-079 (mezagitamab) is a potent monoclonal antibody developed to target CD38, a surface protein abundantly expressed on malignant myeloma cells and to a lesser extent on activated NK, T, and B cells. By binding with high affinity to CD38 on myeloma cells within the bone marrow and peripheral tissues, TAK-079 facilitates targeted immune activity. The therapy is designed to achieve a rapid and sustained rise in platelet counts, restoring them to functional levels efficiently. It is currently in Phase III clinical trials for the treatment of Immune Thrombocytopenic Purpura (ITP).
HLX15 - Shanghai Henlius Biotech
HLX15 is a fully human IgG1 monoclonal antibody targeting CD38, developed by Shanghai Henlius Biotech. It is being evaluated for the treatment of multiple myeloma (MM) - a severe and incurable malignancy of clonal plasma cells, recognized as the second most common blood cancer, predominantly affecting older individuals. In February 2025, Henlius entered a licensing agreement with Dr. Reddy's Laboratories, granting the latter exclusive commercialization rights for both subcutaneous and intravenous formulations of HLX15 across 43 countries, including the U.S. and 42 European markets. The therapy is currently in Phase III clinical development for multiple myeloma.
Anti-CD38 antibody Companies
Around 10 or more prominent companies are actively engaged in developing Anti-CD38 antibody-based therapies. Among these, Takeda and Shanghai Henlius Biotech lead the field with their drug candidates currently in the most advanced stage of development - Phase III clinical trials.
DelveInsight's report covers around 75+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Anti-CD38 antibody pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Anti-CD38 antibody Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Anti-CD38 antibody Therapies and Key Companies: Anti-CD38 antibody Clinical Trials and advancements [https://www.delveinsight.com/report-store/anti-cd38-antibody-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Anti-CD38 antibody Pipeline Therapeutic Assessment
- Anti-CD38 antibody Assessment by Product Type
- Anti-CD38 antibody By Stage
- Anti-CD38 antibody Assessment by Route of Administration
- Anti-CD38 antibody Assessment by Molecule Type
Download Anti-CD38 antibody Sample report to know in detail about the Anti-CD38 antibody treatment market @ Anti-CD38 antibody Therapeutic Assessment [https://www.delveinsight.com/sample-request/anti-cd38-antibody-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Anti-CD38 antibody Current Treatment Patterns
4. Anti-CD38 antibody - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Anti-CD38 antibody Late-Stage Products (Phase-III)
7. Anti-CD38 antibody Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Anti-CD38 antibody Discontinued Products
13. Anti-CD38 antibody Product Profiles
14. Anti-CD38 antibody Key Companies
15. Anti-CD38 antibody Key Products
16. Dormant and Discontinued Products
17. Anti-CD38 antibody Unmet Needs
18. Anti-CD38 antibody Future Perspectives
19. Anti-CD38 antibody Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Anti-CD38 antibody Pipeline Reports Offerings [https://www.delveinsight.com/report-store/anti-cd38-antibody-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=anticd38-antibody-clinical-companies-therapeutic-assessment-therapies-treatment-algorithm-pipeline-analysis-imab-biopharma-takeda-hlx15-ancora-biotech-casi-pharmaceuticals]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Anti-CD38 Antibody Clinical, Companies, Therapeutic Assessment, Therapies, Treatment Algorithm, Pipeline Analysis | I-MAB Biopharma, Takeda, HLX15, Ancora Biotech, CASI Pharmaceuticals here
News-ID: 4241460 • Views: …
More Releases from ABNewswire
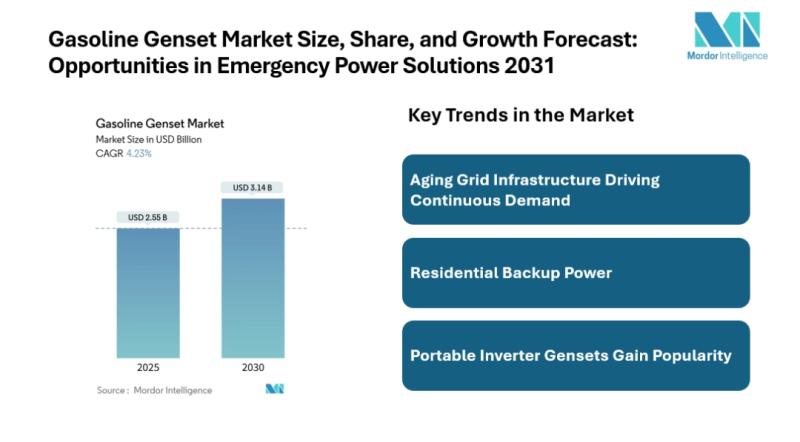
Gasoline Genset Market Size to rise to $ 3.14 Billion by 2030 | Standby and Port …
Mordor Intelligence has published a new report on the Gasoline Genset Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
The global gasoline genset market size estimated at USD 2.55 billion in 2025 and projected to reach USD 3.14 billion by 2030. This represents a CAGR of 4.23% 2025-2030.
The demand is anchored by ongoing vulnerabilities in power grids, increasing urban construction activity in emerging economies, and a growing…

Tofu Market Size to Reach USD 3.77 Billion by 2031 as Flexitarian Diets and Prod …
Mordor Intelligence has published a comprehensive analysis of the tofu market, highlighting long-term growth drivers, evolving consumer trends, and competitive positioning through 2031.
Tofu Market Forecast, Size, and Growth Outlook
According to a research report by Mordor Intelligence, the global tofu market size [https://www.mordorintelligence.com/industry-reports/tofu-market?utm_source=abnewswire] is projected to grow from USD 2.08 billion in 2026 to USD 3.77 billion by 2031, reflecting strong tofu market growth during the forecast period. This expansion is…

Silicone Gel Market Growth at 5% CAGR Across Key End-Use Industries 2025 to 2030 …
Mordor Intelligence has published a new report on the Silicone Gel Market, offering a comprehensive analysis of trends, growth drivers, and future projections.
The Silicone Gel Market is projected to grow steadily over the forecast period, with a CAGR of 5% from 2025 to 2030, reflecting increasing demand across key end-use industries. The market has shown consistent expansion since 2019, driven by applications in electrical and electronics, healthcare, and cosmetics. Silicone…
GLP-1 SuperDefender: The Revolutionary Oral Protection Appliance Against "Ozempi …
Los Angeles, California - Feb 18, 2026 - As medications like Ozempic Registered , Wegovy Registered , and Mounjaro Registered continue to reshape the future of weight management and diabetes treatment, millions of patients are unknowingly facing a growing dental crisis. Reports of acid reflux, dry mouth, and teeth grinding are rising among GLP-1 medication users, leading to irreversible enamel erosion and tooth loss; Collectively known as "Ozempic Teeth".
In response…
More Releases for CD38
DARZALEX Market Set to Maintain Leadership in Multiple Myeloma Therapy with Stro …
New York, USA - DelveInsight's latest report, "DARZALEX Market Size, Forecast, and Market Insight - 2032," provides a comprehensive analysis of DARZALEX (daratumumab) market performance, competitive landscape, and future growth projections across the seven major markets (7MM): the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan. The report offers key product insights, market dynamics, competitor analysis, regulatory milestones, and forecasted sales trends from 2019 to…
CD38 Monoclonal Antibodies Market Size, Clinical Trials, Product Pipelines and I …
CD38 Monoclonal Antibodies Market Size is estimated to be $7550 million in 2024 and is expected to grow at an average yearly rate of around 5% during the timeframe (2025-2032).
What is CD38 Monoclonal Antibodies and what are the growth drivers of CD38 Monoclonal Antibodies Market?
CD38 monoclonal antibodies are a class of targeted therapeutic agents designed to bind specifically to the CD38 protein expressed on the…
Multiple Myeloma Pipeline Appears Robust With 75+ Key Pharma Companies Actively …
DelveInsight's, "Multiple Myeloma Pipeline Insights 2025" report provides comprehensive insights about 75+ Multiple Myeloma Companies and 80+ pipeline drugs in the Multiple Myeloma pipeline landscape. It covers the Multiple Myeloma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Multiple Myeloma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore the comprehensive insights…
Multiple Myeloma Clinical Trials and Studies: EMA, PDMA, FDA Approvals, Mechanis …
DelveInsight's, "Multiple Myeloma Pipeline Insights" report provides comprehensive insights about 75+ Multiple Myeloma Companies and 80+ pipeline drugs in the Multiple Myeloma pipeline landscape. It covers the Multiple Myeloma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Multiple Myeloma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore the comprehensive insights by…
Multiple Myeloma Pipeline Outlook 2024 | Sydnexis, Sunhawk Vision Biotech, Vylum …
DelveInsight's, "Multiple Myeloma Pipeline Insights 2024" report provides comprehensive insights about 75+ Multiple Myeloma Companies and 80+ pipeline drugs in the Multiple Myeloma pipeline landscape. It covers the Multiple Myeloma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Multiple Myeloma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Discover the latest drugs…
Anti-CD38 Antibody Market 2020 Research Report – ImmunoGen, Genmab, MorphoSys, …
Anti-CD38 Antibody -Pipeline Insight, 2020
The report will make detailed analysis mainly on in-depth research on the development environment, Market size, development trend, operation situation and future development trend of Anti-CD38 Antibody Market on the basis of stating current situation of the industry in 2020.
This is a latest report, covering the current COVID-19 impact on the market. The pandemic of Coronavirus (COVID-19) has affected every aspect of life globally. This has…
