Press release
Ovarian Cancer Clinical Trials, Companies, Therapeutic Assessment, Emerging Therapies, Treatment Algorithm, and Pipeline Analysis
Ovarian Cancer companies are Allarity Therapeutics, OSE Immunotherapeutic, Cristal Therapeutics, Bristol-Myers Squibb, Ono Pharmaceuticals, Merck & Co., Aravive Biologics, Mersana Therapeutics, Clovis Oncology, Verastem Oncology, Gradalis, AbbVie, Elevation oncology, OncoQuest Pharmaceuticals (CanariaBio), Alkermes, Hoffman-la Roche, AstraZeneca, MSD, GlaxoSmithKline, IMV, Corcept Therapeutics, and others.Ovarian Cancer Pipeline constitutes 180+ key companies continuously working towards developing 200+ Ovarian Cancer treatment therapies, analyzes DelveInsight.
Ovarian Cancer Overview:
Ovarian cancer is the deadliest gynecological cancer and the fifth leading cause of death among women overall. Late-stage diagnosis is common, resulting in poor outcomes. Current screening methods, including cancer antigen-125 (CA-125) assays, have limited effectiveness in reducing morbidity or mortality.
Epithelial ovarian cancer is the most common form, with four main histological types: serous, endometrioid, clear cell, and mucinous tumors, as well as rarer types like Brenner and seromucinous tumors. Based on biology and prognosis, ovarian cancer is classified into two subtypes:
- Type I tumors: Include low-grade serous, endometrioid, clear cell, mucinous, Brenner, and seromucinous carcinomas. They generally originate from borderline tumors, are detected early, and have a favorable prognosis. These tumors exhibit low proliferative activity and minimal genetic instability.
- Type II tumors: Include high-grade serous carcinoma, carcinosarcoma, and undifferentiated carcinoma. They arise from serous tubal intraepithelial carcinoma, are typically aggressive, high-grade, and diagnosed at advanced stages. They show rapid progression, significant chromosomal instability, and frequent mutations in the p53 gene.
Type II tumors are more aggressive and lethal than Type I, highlighting the need for improved early detection and treatment strategies.
Request for a detailed insights report on Ovarian Cancer pipeline insights @ https://www.delveinsight.com/report-store/ovarian-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr
"Ovarian Cancer Pipeline Insight 2025" report by DelveInsight provides a comprehensive analysis of the ongoing clinical development activities and growth prospects across the Ovarian Cancer Therapeutics Market.
Key Takeaways from the Ovarian Cancer Pipeline Report
*
DelveInsight's Ovarian Cancer pipeline report depicts a robust space with 180+ active players working to develop 200+ pipeline therapies for Ovarian Cancer treatment.
*
In May 2025, Allarity Therapeutics concluded a Phase II trial for stenoparib (2X-121) after observing significant clinical benefits in earlier trial phases. The company now aims to launch a follow-up trial designed to expedite regulatory submission for stenoparib.
*
Key Ovarian Cancer companies such as Allarity Therapeutics, OSE Immunotherapeutic, Cristal Therapeutics, Bristol-Myers Squibb, Ono Pharmaceuticals, Merck & Co., Aravive Biologics, Mersana Therapeutics, Clovis Oncology, Verastem Oncology, Gradalis, AbbVie, Elevation oncology, OncoQuest Pharmaceuticals (CanariaBio), Alkermes, Hoffman-la Roche, AstraZeneca, MSD, GlaxoSmithKline, IMV, Corcept Therapeutics, and others are evaluating new drugs for Ovarian Cancer to improve the treatment landscape.
*
Promising Ovarian Cancer pipeline therapies in various stages of development include Atezolizumab, Tisotumab, SON-1010, DS-6000a, and others.
Ovarian Cancer Pipeline Analysis
The report provides insights into:
*
The report provides detailed insights into the key companies that are developing therapies in the Ovarian Cancer Market.
*
The report also evaluates different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Ovarian Cancer treatment.
*
It analyzes the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
It navigates the emerging drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement, and financing details for future advancement of the Ovarian Cancer market.
Download our free sample page report on Ovarian Cancer pipeline insights [https://www.delveinsight.com/sample-request/ovarian-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Ovarian Cancer Emerging Drugs
*
Atezolizumab: Genentech
*
Tisotumab Vedotin: Genmab
*
SON-1010: Sonnet Biotherapeutics
*
DS-6000a: Daiichi Sankyo Company
Ovarian Cancer Companies
Over 180 major companies are actively developing therapies for ovarian cancer. Among them, Genentech stands out with its drug candidates in the most advanced stage of development, Phase III.
DelveInsight's report covers around 200+ products under different phases of clinical development like
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I) along with the details of
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Ovarian Cancer pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
*
Intravenous
*
Subcutaneous
*
Oral
*
Intramuscular
Ovarian Cancer Products have been categorized under various Molecule types such as
*
Monoclonal antibody
*
Small molecule
*
Peptide
Download Sample Pages to Get an in-depth Assessment of the Emerging Ovarian Cancer Therapies and Key Companies: Ovarian Cancer Clinical Trials and advancements [https://www.delveinsight.com/report-store/ovarian-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Ovarian Cancer Pipeline Therapeutic Assessment
- Ovarian Cancer Assessment by Product Type
- Ovarian Cancer By Stage
- Ovarian Cancer Assessment by Route of Administration
- Ovarian Cancer Assessment by Molecule Type
Download Ovarian Cancer Sample report to know in detail about the Ovarian Cancer treatment market @ Ovarian Cancer Therapeutic Assessment [https://www.delveinsight.com/sample-request/ovarian-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Content
1. Report Introduction
2. Executive Summary
3. Ovarian Cancer Current Treatment Patterns
4. Ovarian Cancer - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Ovarian Cancer Late-Stage Products (Phase-III)
7. Ovarian Cancer Mid-Stage Products (Phase-II)
8. Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Ovarian Cancer Discontinued Products
13. Ovarian Cancer Product Profiles
14. Ovarian Cancer Key Companies
15. Ovarian Cancer Key Products
16. Dormant and Discontinued Products
17. Ovarian Cancer Unmet Needs
18. Ovarian Cancer Future Perspectives
19. Ovarian Cancer Analyst Review
20. Appendix
21. Report Methodology
Request the Sample PDF to Get Detailed Insights About the Ovarian Cancer Pipeline Reports Offerings [https://www.delveinsight.com/report-store/ovarian-cancer-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=ovarian-cancer-clinical-trials-companies-therapeutic-assessment-emerging-therapies-treatment-algorithm-and-pipeline-analysis]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Ovarian Cancer Clinical Trials, Companies, Therapeutic Assessment, Emerging Therapies, Treatment Algorithm, and Pipeline Analysis here
News-ID: 4206179 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
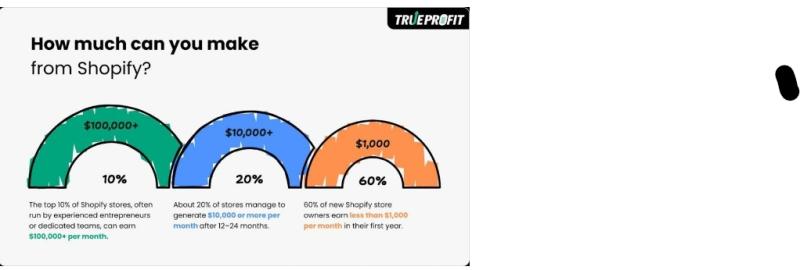
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Ovarian
Ovarian Cancer Diagnostics Market Size: 2033 Statistics
The Ovarian Cancer Diagnostics market is expected to grow from an estimated USD 4814.5 million in 2024 to USD 7795.1 million in 2033, at a CAGR of 5.50%.
The ovarian cancer diagnostics market plays a critical role in the early detection and management of ovarian cancer, one of the most common and lethal gynecological cancers worldwide. Ovarian cancer is often diagnosed at an advanced stage due to the lack of noticeable…
Ovarian Cancer Market Size, Future Trends
𝐔𝐒𝐀, 𝐍𝐞𝐰 𝐉𝐞𝐫𝐬𝐞𝐲- The global Ovarian Cancer Market is expected to record a CAGR of XX.X% from 2024 to 2031 In 2024, the market size is projected to reach a valuation of USD XX.X Billion. By 2031 the valuation is anticipated to reach USD XX.X Billion.
The impact of manufacturers on the market is significant across various industries, influencing supply chains, consumer choices, and economic growth. Manufacturers are key players in…
Polycystic Ovarian Syndrome Treatment Market Report
The findings reviewed by GME stated that the Global Polycystic Ovarian Syndrome Treatment Market will expand with a CAGR value of 5.2 percent from 2021 to 2026. The rising incidence of PCOS increased understanding among a group of patients, and the growing acceptance of standard treatment boosts the PCOS treatment industry growth over the projected timeframe. Furthermore, the research and development of PCOS are predicted to aid in market growth.
Browse…
How deadly is Ovarian Cancer?
Ovarian Drugs and Treatment Market describes its growth, size, share, Forecast and trends to 2025
Ovarian cancer is a cancer that forms in or on an ovary. It results in abnormal cells that have the ability to invade or spread to other parts of the body.
The global Ovarian Cancer Treatment Drugs market is valued at xx million US$ in 2018 and will reach xx million US$ by the end of 2025,…
Polycystic Ovarian Syndrome Treatment Market - Regional Analysis
The organic and inorganic strategies by the market players will boost the polycystic ovarian syndrome treatment market. For instance, in 2016, Millendo Therapeutics, Inc., a biopharmaceutical company, entered into an exclusive license agreement with AstraZeneca for the development and commercialization of phase II entered AZD4901, a candidate product for the treatment of polycystic ovarian syndrome. Moreover, in 2017, Astellas Pharma Inc. completed its acquisition of Ogeda SA (formerly Euroscreen S.A.).…
Ovarian Cancer - Heat Map and Analysis
ReportsWorldwide has announced the addition of a new report title Ovarian Cancer - Heat Map and Analysis to its growing collection of premium market research reports.
Ovarian Cancer (OC) is the fifth most common malignancy diagnosed in women, and the most common gynecological cancer, with an estimated 225,000 new cases diagnosed globally in 2008. Rates are particularly high in developed countries, and especially in Northern Europe. However, substantial improvements in…
