Press release
Helicobacter Pylori Infections Market Outlook 2034 - Clinical Trials, Market Size, Medication, Prevalence, Companies by DelveInsight
Helicobacter Pylori Infections companies working in the market are Servatus Biopharmaceuticals, Cinclus Pharma Holding AB, Crestone, EpiVax, Iguana Biotechnology, ImevaX GmbH, ImmunoBiology, Luoxin Pharmaceuticals, Nexbiome Therapeutics, Recce Pharmaceuticals, RedHill Biopharma Ltd, SCG Cell Therapy Pte Ltd, Shanghai High-Tech Bioengineering Co Ltd, Takeda Pharmaceutical, TenNor Therapeutics, Trio Medicines, Xiamen Encheng Pharmaceutical Co Ltd and others.Helicobacter Pylori Infections Market Summary
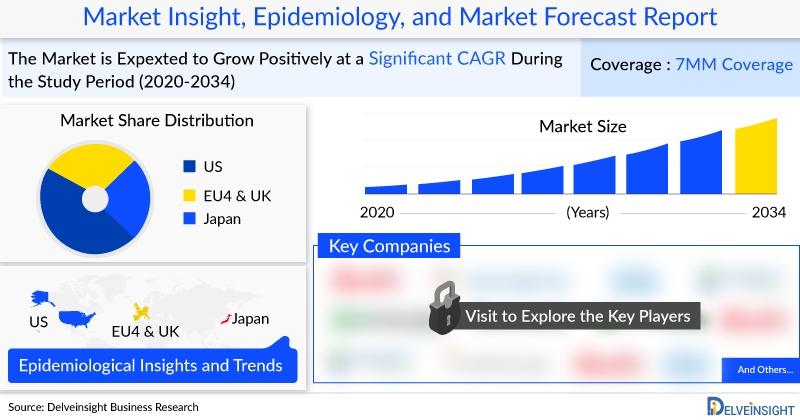
In 2022, the U.S. held the largest share of the Helicobacter pylori (H. pylori) infection market among the 7MM, valued at about USD 1,064.8 million, and is projected to grow further by 2034. Clarithromycin triple therapy, a combination of PPI, clarithromycin, and either amoxicillin or metronidazole, remains the most widely used, generating USD 351 million in revenue in 2022. The market expansion is fueled by rising prevalence, though antibiotic resistance poses a growing challenge. Current therapies, including PREVPAC, PYLERA, and generics, aim at bacterial eradication to heal ulcers and prevent relapses. New entrants like RedHill's TALICIA and Phathom's VOQUEZNA (approved in 2022 and relaunched in 2023) are reshaping the treatment landscape. Ongoing research is driving innovation, with promising pipeline drugs like TenNor's Rifasutenizole (TNP-2198), expected to launch in 2027, offering dual-action efficacy and reduced resistance risk, potentially intensifying competition in the U.S. H. pylori infection market.
(Albany, USA) DelveInsight's "Helicobacter Pylori Infections Market Insights, Epidemiology, and Market Forecast-2034" report provides a comprehensive overview of H. pylori infections, covering historical and projected epidemiology and market trends across the US, EU5, and Japan. It details current treatment practices, emerging therapies, individual drug market shares, and market size forecasts from 2020-2034. The report also examines treatment algorithms, key drivers, barriers, and unmet needs to highlight opportunities and assess the market's growth potential.
Request for a Free Sample Report @ [https://www.delveinsight.com/report-store/helicobacter-pylori-infections-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Some facts of the Helicobacter Pylori Infections Market Report are:
* According to DelveInsight, Helicobacter Pylori Infections market size is expected to grow at a decent CAGR by 2034.
* The total Helicobacter pylori Infection market size in the 7MM was approximately USD 2,672.5 million in 2022 and is projected to increase during the forecast period (2023-2034).
* Leading Helicobacter Pylori Infections companies working in the market are Servatus Biopharmaceuticals, Cinclus Pharma Holding AB, Crestone, EpiVax, Iguana Biotechnology, ImevaX GmbH, ImmunoBiology, Luoxin Pharmaceuticals, Nexbiome Therapeutics, Recce Pharmaceuticals, RedHill Biopharma Ltd, SCG Cell Therapy Pte Ltd, Shanghai High-Tech Bioengineering Co Ltd, Takeda Pharmaceutical, TenNor Therapeutics, Trio Medicines, Xiamen Encheng Pharmaceutical Co Ltd and others.
* Key Helicobacter Pylori Infections Therapies expected to launch in the market are Rifasutenizole (TNP-2198), SVT1C4610, Vonoprazan, SQ 109, Tegoprazan, IMX 101, CRS3123, and many others.
* In March 2025, TenNor Therapeutics Inc announced results of a Phase 3 Clinical Trial to Evaluate the Efficacy and Safety of Rifasutenizol (TNP 2198) in Combination With Rabeprazole and Amoxicillin in the Primary Treatment of Participants With H. Pylori Infection
* In February 2025, TenNor Therapeutics Inc. announced results of a Phase 1, Single-center, Double-blind, Placebo-controlled Study to Evaluate Pharmacokinetics (PK) and Preliminary Efficacy of Multiple Oral Administrations of TNP-2092 Capsules in Healthy Subjects With Helicobacter Pylori Infection.
* In December 2024, Daewoong Pharmaceutical Co. LTD announced results of a Multi-center, Randomized, Double-blind, Parallel-group, Active-controlled, Phase III Clinical Trial to Evaluate the Efficacy and Safety of DWP14012-based Triple Therapy in Eradication of Helicobacter Pylori
* In November 2024, TenNor Therapeutics has revealed that its antibiotic candidate, rifasutenizol, has successfully met all primary endpoints in a Phase III trial, outperforming the current standard of care (SoC). The multicenter, randomized, double-blind, controlled trial (NCT05857163) demonstrated that rifasutenizol triple therapy achieved an eradication rate of over 90%, surpassing the effectiveness of bismuth-containing quadruple therapy (BQT) in treating Helicobacter pylori (H. pylori) infection, a prevalent bacterial infection of the stomach often responsible for stomach ulcers.
* In March 2024, The stool antigen test (SAT) and serum Helicobacter pylori (H. pylori) IgG antibody assays are highly effective in diagnosing H. pylori infections and differentiating between acute and chronic cases. This study aimed to assess the diagnostic value of serum H. pylori IgG antibodies and SAT in detecting H. pylori infections among patients with chronic H. pylori infection in Ibb Governorate, Yemen. A total of 200 patients, confirmed to have H. pylori infection through positive results from the serum immunochromatographic antibody test, were selected for further confirmation using serum H. pylori IgG antibodies and SAT at various hospitals and gastroenterology and hepatology clinics in Ibb Governorate.
* In September 2022, the US FDA granted linaprazan glurate a QIDP for the treatment of H. pylori infection.
* Currently a Phase III clinical trial is being conducted in China for the treatment of H. pylori infection to explore screening-eradication strategy on large scale so as to prevent gastric cancer in populations with relatively high incidence of gastric cancer. The drug has received IND approval and Qualified Infectious Disease Product (QIDP designation) from the US FDA.
* In 2022, the US FDA approved Phathom Pharmaceuticals, P-CAB combination, VOQUEZNA. as triple and dual pak. It contains vonoprazan, a PCAB, with amoxicillin, a penicillin class antibacterial with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms, and clarithromycin, a semi-synthetic macrolide antimicrobial for oral use, while, the DUAL PAK contains vonoprazan and amoxicillin only.
Helicobacter Pylori Infections Overview
Helicobacter pylori (H. pylori) is a type of bacteria that infects the stomach lining, causing various gastrointestinal disorders. It is one of the most common bacterial infections worldwide, with an estimated two-thirds of the global population infected. H. pylori is primarily transmitted through contaminated food, water, or direct contact with saliva or feces of infected individuals. While many infected individuals remain asymptomatic, H. pylori infection can lead to various gastrointestinal conditions, including gastritis (inflammation of the stomach lining), peptic ulcers (sores in the lining of the stomach or small intestine), and even stomach cancer in some cases. Symptoms of H. pylori infection may include abdominal pain, bloating, nausea, vomiting, and loss of appetite. Diagnosis typically involves a combination of tests, such as breath tests, stool tests, blood tests, and endoscopic examinations. Treatment for H. pylori infection usually involves a combination of antibiotics and acid-suppressing medications to eradicate the bacteria and alleviate symptoms. Early detection and treatment of H. pylori infection are essential to prevent complications and reduce the risk of developing more serious gastrointestinal diseases.
Do you know what will be the Helicobacter Pylori Infections market share in 7MM by 2034 @ Helicobacter Pylori Infections Market Outlook [https://www.delveinsight.com/sample-request/helicobacter-pylori-infections-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Helicobacter Pylori Infections Market Outlook
In 2022, the total H. pylori infection market size across the 7MM was about USD 2,672.5 million and is projected to grow through 2034. The U.S. led with USD 1,064.8 million, driven by awareness and emerging therapies, while EU4 and the UK together accounted for USD 1,028.3 million (~38% of the 7MM market). Within Europe, France held the largest share (USD 237.3 million), followed by Germany (USD 221.2 million), while the UK had the lowest. Japan contributed USD 579.4 million, ranking second overall, with strong expected growth by 2034.
The Helicobacter pylori (H. pylori) infection market is segmented by therapies, with key approved products including TALICIA and VOQUEZNA (US only), OMECLAMOX-Pak and PYLERA (US and EU), and VONOSAP, VONOPION, TAKECAB, RABECURE, and RABEFINE (Japan only). Pipeline therapies such as rifasutenizole (TNP-2198) are under evaluation.
Helicobacter Pylori Infections Epidemiology
The Helicobacter Pylori Infections epidemiology section provides insights into the historical and current Helicobacter Pylori Infections patient pool and forecasted trends for seven individual major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the Helicobacter Pylori Infections market report also provides the diagnosed patient pool, trends, and assumptions.
Interested to know how the emerging diagnostic approaches will be contributing in increased Helicobacter Pylori Infections diagnosed prevalence pool? Download report @ Helicobacter Pylori Infections Patient Pool Forecast [https://www.delveinsight.com/report-store/helicobacter-pylori-infections-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Helicobacter Pylori Infections Drugs Uptake
Rifasutenizole (TNP-2198): TenNor Therapeutics
Rifasutenizole (TNP-2198) is a novel multitarget drug conjugate exhibiting a synergistic mechanism of action, providing potent bactericidal activity against drug-resistant H. pylori strains while maintaining a very low spontaneous resistance rate. Its therapeutic potential extends to infections including H. pylori , bacterial vaginosis, and Clostridioides difficile .
TNP-2198 has received support from China's National Major New Drug Innovation grant and holds IND approval and QIDP designation from the U.S. FDA for H. pylori treatment. The program has completed five clinical trials in China and is currently in a multi-center, randomized, double-blind, bismuth-containing quadruple therapy-controlled Phase III study, designed to evaluate a safe, efficient, and simplified eradication regimen compatible with urea breath test (UBT) monitoring. This strategy may enable large-scale H. pylori screening and eradication to reduce gastric cancer incidence in high-risk populations. Additionally, TNP-2198 is under investigation in Phase II trials for bacterial vaginosis and C. difficile infection.
Helicobacter Pylori Infections Competitive Landscape
* TALICIA (RHB-105): RedHill Biopharma
* VOQUEZNA TRIPLE PAK and VOQUEZNA DUAL PAK: Phathom Pharmaceuticals
* TAKECAB (vonoprazan): Takeda Pharmaceutical/Otsuka Pharmaceutical
* PYLERA: Juvise Pharmaceuticals/AbbVie
* Rifasutenizole (TNP-2198): TenNor Therapeutics
* BGA-1901: Nexbiome therapeutics
* Linaprazan glurate: Cinclus Pharma
* Esomeprazole: Elpen Pharmaceutical
* TNP-2198: TenNor Therapeutics
* Vonoprazan: Phathom Pharmaceuticals
* Tegoprazan: HK inno.N Corporation
* Levofloxacin: Deva Holding A.S.
* Lansoprazole (Lanton): Meridian Bioscience, Inc.
* Vonoprazan: Takeda
* RHB-105: RedHill Biopharma
* rabeprazole sodium: Janssen Cilag Pharmaceutica
* TAK-438: Takeda
* Ilaprazole + Amoxicillin: Il-Yang Pharm
* Omeprazole, amoxicillin, clarithromycin: Forest Laboratories
Download report to know which TOP 3 therapies will be capturing the largest Helicobacter Pylori Infections market share by 2034? Click here @ Helicobacter Pylori Infections Drugs and Therapies [https://www.delveinsight.com/sample-request/helicobacter-pylori-infections-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Helicobacter Pylori Infections Therapeutics Assessment
Major key companies are working proactively in the Helicobacter Pylori Infections Therapeutics market to develop novel therapies which will drive the Helicobacter Pylori Infections treatment markets in the upcoming years are Servatus Biopharmaceuticals, Cinclus Pharma Holding AB, Crestone, EpiVax, Iguana Biotechnology, ImevaX GmbH, ImmunoBiology, Luoxin Pharmaceuticals, Nexbiome Therapeutics, Recce Pharmaceuticals, RedHill Biopharma Ltd, SCG Cell Therapy Pte Ltd, Shanghai High-Tech Bioengineering Co Ltd, Takeda Pharmaceutical, TenNor Therapeutics, Trio Medicines, Xiamen Encheng Pharmaceutical Co Ltd, and others.
Do you know how Rifasutenizole (TNP-2198) and SVT1C4610 market launch will be impacting the Helicobacter Pylori Infections market CAGR? Download sample report @ Helicobacter Pylori Infections Therapeutics Market [https://www.delveinsight.com/sample-request/helicobacter-pylori-infections-market?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=apr]
Helicobacter Pylori Infections Report Key Insights
1. Helicobacter Pylori Infections Patient Population
2. Helicobacter Pylori Infections Market Size and Trends
3. Key Cross Competition in the Helicobacter Pylori Infections Market
4. Helicobacter Pylori Infections Market Dynamics (Key Drivers and Barriers)
5. Helicobacter Pylori Infections Market Opportunities
6. Helicobacter Pylori Infections Therapeutic Approaches
7. Helicobacter Pylori Infections Pipeline Analysis
8. Helicobacter Pylori Infections Current Treatment Practices/Algorithm
9. Impact of Emerging Therapies on the Helicobacter Pylori Infections Market
Table of Contents
1. Key Insights
2. Executive Summary
3. Helicobacter Pylori Infections Competitive Intelligence Analysis
4. Helicobacter Pylori Infections Market Overview at a Glance
5. Helicobacter Pylori Infections Disease Background and Overview
6. Helicobacter Pylori Infections Patient Journey
7. Helicobacter Pylori Infections Epidemiology and Patient Population
8. Helicobacter Pylori Infections Treatment Algorithm, Current Treatment, and Medical Practices
9. Helicobacter Pylori Infections Unmet Needs
10. Key Endpoints of Helicobacter Pylori Infections Treatment
11. Helicobacter Pylori Infections Marketed Products
12. Helicobacter Pylori Infections Emerging Therapies
13. Helicobacter Pylori Infections Seven Major Market Analysis
14. Attribute Analysis
15. Helicobacter Pylori Infections Market Outlook (7 major markets)
16. Helicobacter Pylori Infections Access and Reimbursement Overview
17. KOL Views on the Helicobacter Pylori Infections Market
18. Helicobacter Pylori Infections Market Drivers
19. Helicobacter Pylori Infections Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=helicobacter-pylori-infections-market-outlook-2034-clinical-trials-market-size-medication-prevalence-companies-by-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/primary-research-services
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Helicobacter Pylori Infections Market Outlook 2034 - Clinical Trials, Market Size, Medication, Prevalence, Companies by DelveInsight here
News-ID: 4194883 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Helicobacter
Helicobacter Pylori Diagnostic Market Size to Expand Lucratively by 2031
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Helicobacter Pylori Diagnostic Market Size, Share & Trends Analysis Report By Technology (Immunoassays, POC, Molecular Diagnostics), By End User (Hospitals, Diagnostics Laboratories, Clinics)- Market Outlook And Industry Analysis 2031"
The Global Helicobacter Pylori Diagnostic Market is estimated to reach over USD 994.69 million by 2031, exhibiting a CAGR of 6.20% during the forecast period.
Get Free Access…
Helicobacter Pylori Testing Market 2024 Size, Status and Global Outlook |
Helicobacter Pylori Testing Market Overview:
A lot of factors, such as geographic growth, segmentation, and market size by value and volume, are taken into account in the SkyQuest Technology Group research to provide a full and accurate analysis of the global Helicobacter Pylori Testing market. This outstanding research study was created specifically to provide the most latest data on significant aspects of the global Helicobacter Pylori Testing Industry. Numerous…
Helicobacter Pylori Diagnostic Market Research Report 2023 | InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Helicobacter Pylori Diagnostic Market Size, Share & Trends Analysis Report By Technology (Immunoassays, POC, Molecular Diagnostics), By End User (Hospitals, Diagnostics Laboratories, Clinics)- Market Outlook And Industry Analysis 2031"
The Global Helicobacter Pylori Diagnostic Market is estimated to reach over USD 994.69 million by 2031, exhibiting a CAGR of 6.20% during the forecast period.
Request…
Helicobacter Pylori Diagnostics Market Size - Forecast to 2026
The findings reviewed by GME stated that the Global Helicobacter Pylori Diagnostics Market would grow at a CAGR value of 7.4 percent from 2021 to 2026. With drastically increasing advancements and less complex diagnostic processes, the reliability and demand for helicobacter pylori diagnostics are increasing. Also, most developing countries have unsanitary water services and facilities, along with a lack of awareness about personal care; the helicobacter pylori bacterial infection is…
Helicobacter Pylori Testing Market: Competitive Dynamics & Global Outlook 2025
Market Research Report Store offers a latest published report on Helicobacter Pylori Testing Market Analysis and Forecast 2019-2025 delivering key insights and providing a competitive advantage to clients through a detailed report.
According to this study, over the next five years the Helicobacter Pylori Testing market will register a 7.5% CAGR in terms of revenue, the global market size will reach $ 781.6 million by 2025, from $ 586 million in…
Global Helicobacter Pylori Testing Market Growth 2019-2024
LP INFORMATION offers a latest published report on Helicobacter Pylori Testing Market Analysis and Forecast 2019-2025 delivering key insights and providing a competitive advantage to clients through a detailed report.
According to this study, over the next five years the Helicobacter Pylori Testing market will register a xx% CAGR in terms of revenue, the global market size will reach US$ xx million by 2024, from US$ xx million in 2019.…