Press release
Beta Thalassemia Pipeline 2025: Latest FDA Approvals, Clinical Trials, and Emerging Therapies Assessment by DelveInsight | Ionis Pharmaceuticals, Inc., Agios Pharma, Silence Therapeutics, Phoenicia
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Beta Thalassemia pipeline constitutes 22+ key companies continuously working towards developing 22+ Beta Thalassemia treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight."Beta Thalassemia Pipeline Insight, 2025 [https://www.delveinsight.com/sample-request/beta-thalassemia-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Beta Thalassemia Market.
The Beta Thalassemia Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
Some of the key takeaways from the Beta Thalassemia Pipeline Report:
*
Companies across the globe are diligently working toward developing novel Beta Thalassemia treatment therapies with a considerable amount of success over the years.
*
Beta Thalassemia companies working in the treatment market are Agios Pharmaceuticals, Vertex Pharmaceuticals, Pharmacosmos, Editas Medicine, HemaQuest Pharmaceuticals, Acceleron Pharma, Bristol-Myers Squibb, Hoffmann-La Roche, Sanofi, Novartis, EmeraMed, Pharmacosmos A/S, Celgene, DisperSol Technologies, LLC, Agios Pharmaceuticals, Shire, and others, are developing therapies for the Beta Thalassemia treatment
*
Emerging Beta Thalassemia therapies in the different phases of clinical trials are- PYRUKYND (mitapivat), CTX001, SP-420, EDIT-301, dimethylbutyrate, ACE-536, Luspatercept, Bitopertin, Deferitrin (GT56-252), ICL670, Emeramide, SP-420, Luspatercept, DST-0509, Mitapivat, SPD602, and others are expected to have a significant impact on the Beta Thalassemia market in the coming years.
*
In July 2025, Vertex Pharmaceuticals' Casgevy (exagamglogene autotemcel) demonstrated sustained benefits in sickle cell disease (SCD) for 5.5 years and in transfusion-dependent beta-thalassemia (TDT) for six years. Data from three ongoing Phase III trials-CLIMB-111 (NCT03655678), CLIMB-121 (NCT03745287), and CLIMB-131 (NCT04208529)-show that all 45 SCD patients treated with Casgevy achieved at least 12 consecutive months without hospitalization, averaging 36.1 months. Additionally, 95.6% remained free from vaso-occlusive crises (VOCs) for at least 12 months, with an average duration of 35 months. Among TDT patients, 98.2% of 55 participants achieved transfusion independence for at least 12 consecutive months, maintaining a weighted average hemoglobin of greater than or equal to 9 g/dL, with a mean duration of 40.5 months.
*
In June 2025, Vertex Pharmaceuticals' Casgevy (exagamglogene autotemcel) has shown long-term efficacy, with benefits lasting 5.5 years in sickle cell disease (SCD) and six years in transfusion-dependent beta-thalassemia (TDT). Results from three ongoing Phase III studies-CLIMB-111 (NCT03655678), CLIMB-121 (NCT03745287), and CLIMB-131 (NCT04208529)-revealed that all 45 SCD patients achieved at least 12 consecutive months free from hospitalization, with an average of 36.1 months. Moreover, 95.6% of these patients remained free from vaso-occlusive crises (VOCs) for at least a year, averaging 35 months. In TDT, 98.2% of 55 patients reached transfusion independence for at least 12 consecutive months, maintaining hemoglobin levels of greater than or equal to 9 g/dL, with a mean duration of 40.5 months.
*
In January 2025, Agios Pharmaceuticals (AGIO) announced that the FDA has accepted its supplemental new drug application (sNDA) to expand the label of Pyrukynd, its sole marketed drug, to include the treatment of thalassemia.
*
In January 2025, YolTech Therapeutics is preparing to launch a clinical trial for YOLT-204, its in vivo gene-editing therapy designed to treat transfusion-dependent beta-thalassemia (TDT). TDT is a genetic blood disorder caused by mutations in the beta-globin gene, leading to reduced or absent hemoglobin production. Patients with this condition require regular blood transfusions to manage anemia and prevent future complications.
Beta Thalassemia Overview
Beta thalassemia is a genetic blood disorder that reduces the production of hemoglobin, a protein in red blood cells that carries oxygen throughout the body. It results from mutations in the HBB gene, which provides instructions for making the beta-globin subunit of hemoglobin. This disorder leads to a deficiency in the beta-globin chains, causing the red blood cells to become malformed and break down prematurely, leading to anemia.
Get a Free Sample PDF Report to know more about Beta Thalassemia Pipeline Therapeutic Assessment-
https://www.delveinsight.com/report-store/beta-thalassemia-pipeline-insight [https://www.delveinsight.com/report-store/beta-thalassemia-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Emerging Beta Thalassemia Drugs Under Different Phases of Clinical Development Include:
*
PYRUKYND (mitapivat): Agios Pharmaceuticals
*
CTX001: Vertex Pharmaceuticals
*
SP-420: Pharmacosmos
*
EDIT-301: Editas Medicine
*
dimethylbutyrate: HemaQuest Pharmaceuticals
*
ACE-536: Acceleron Pharma
*
Luspatercept: Bristol-Myers Squibb
*
Bitopertin: Hoffmann-La Roche
*
Deferitrin (GT56-252): Sanofi
*
ICL670: Novartis
*
Emeramide: EmeraMed
*
SP-420: Pharmacosmos A/S
*
Luspatercept: Celgene
*
DST-0509: DisperSol Technologies, LLC
*
Mitapivat: Agios Pharmaceuticals
*
SPD602: Shire
Beta Thalassemia Route of Administration
Beta Thalassemia pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
*
Oral
*
Parenteral
*
Intravenous
*
Subcutaneous
*
Topical
Beta Thalassemia Molecule Type
Beta Thalassemia Products have been categorized under various Molecule types, such as
*
Monoclonal Antibody
*
Peptides
*
Polymer
*
Small molecule
*
Gene therapy
Beta Thalassemia Pipeline Therapeutics Assessment
*
Beta Thalassemia Assessment by Product Type
*
Beta Thalassemia By Stage and Product Type
*
Beta Thalassemia Assessment by Route of Administration
*
Beta Thalassemia By Stage and Route of Administration
*
Beta Thalassemia Assessment by Molecule Type
*
Beta Thalassemia by Stage and Molecule Type
DelveInsight's Beta Thalassemia Report covers around 22+ products under different phases of clinical development like
*
Late-stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
*
Route of Administration
Further Beta Thalassemia product details are provided in the report. Download the Beta Thalassemia pipeline report to learn more about the emerging Beta Thalassemia therapies [https://www.delveinsight.com/sample-request/beta-thalassemia-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Some of the key companies in the Beta Thalassemia Therapeutics Market include:
Key companies developing therapies for Beta Thalassemia are - Ionis Pharmaceuticals, Inc., Agios Pharmaceuticals, Inc., Silence Therapeutics plc, Phoenicia Biosciences, Shanghai BDgene, Beam Therapeutics, EmeraMed, CRISPR Therapeutics, CSL Vifor, EdiGene (GuangZhou) Inc., Regenacy Pharmaceuticals, Editas Medicine, Fulcrum Therapeutics, Allife Medical Science and Technology, Global Blood Therapeutics, Inc, Orchard Therapeutics, Acceleron Pharma, Disc Medicine, BRL Medicine, Celgene, and others.
Beta Thalassemia Pipeline Analysis:
The Beta Thalassemia pipeline report provides insights into
*
The report provides detailed insights about companies that are developing therapies for the treatment of Beta Thalassemia with aggregate therapies developed by each company for the same.
*
It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Beta Thalassemia Treatment.
*
Beta Thalassemia key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
Beta Thalassemia Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Beta Thalassemia market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Download Sample PDF Report to know more about Beta Thalassemia drugs and therapies [https://www.delveinsight.com/sample-request/beta-thalassemia-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Beta Thalassemia Pipeline Market Strengths
*
Strong pipeline activity with potential Phase III and phase II emerging therapies.
*
Increasing prevalence and awareness of beta thalassemia
Beta Thalassemia Pipeline Market Opportunities
*
Lack of any curative treatment options despite the launch of gene therapies which are subject to patient outcome assessment
*
Improved national blood policies
*
Advancement in genetic engineering because gene therapy has been hypothesized to serve as an effective cure for monogenic blood disorders for several decades
Scope of Beta Thalassemia Pipeline Drug Insight
*
Coverage: Global
*
Key Beta Thalassemia Companies: Agios Pharmaceuticals, Vertex Pharmaceuticals, Pharmacosmos, Editas Medicine, HemaQuest Pharmaceuticals, Acceleron Pharma, Bristol-Myers Squibb, Hoffmann-La Roche, Sanofi, Novartis, EmeraMed, Pharmacosmos A/S, Celgene, DisperSol Technologies, LLC, Agios Pharmaceuticals, Shire, and others
*
Key Beta Thalassemia Therapies: PYRUKYND (mitapivat), CTX001, SP-420, EDIT-301, dimethylbutyrate, ACE-536, Luspatercept, Bitopertin, Deferitrin (GT56-252), ICL670, Emeramide, SP-420, Luspatercept, DST-0509, Mitapivat, SPD602, and others
*
Beta Thalassemia Therapeutic Assessment: Beta Thalassemia current marketed and Beta Thalassemia emerging therapies
*
Beta Thalassemia Market Dynamics: Beta Thalassemia market drivers and Beta Thalassemia market barriers
Request for Sample PDF Report for Beta Thalassemia Pipeline Assessment and clinical trials [https://www.delveinsight.com/sample-request/beta-thalassemia-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]
Table of Contents
1. Beta Thalassemia Report Introduction
2. Beta Thalassemia Executive Summary
3. Beta Thalassemia Overview
4. Beta Thalassemia- Analytical Perspective In-depth Commercial Assessment
5. Beta Thalassemia Pipeline Therapeutics
6. Beta Thalassemia Late Stage Products (Phase II/III)
7. Beta Thalassemia Mid Stage Products (Phase II)
8. Beta Thalassemia Early Stage Products (Phase I)
9. Beta Thalassemia Preclinical Stage Products
10. Beta Thalassemia Therapeutics Assessment
11. Beta Thalassemia Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Beta Thalassemia Key Companies
14. Beta Thalassemia Key Products
15. Beta Thalassemia Unmet Needs
16 . Beta Thalassemia Market Drivers and Barriers
17. Beta Thalassemia Future Perspectives and Conclusion
18. Beta Thalassemia Analyst Views
19. Appendix
20. About DelveInsight
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
Media Contact
Company Name: DelveInsight
Contact Person: Gaurav Bora
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=beta-thalassemia-pipeline-2025-latest-fda-approvals-clinical-trials-and-emerging-therapies-assessment-by-delveinsight-ionis-pharmaceuticals-inc-agios-pharma-silence-therapeutics-phoenicia]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Beta Thalassemia Pipeline 2025: Latest FDA Approvals, Clinical Trials, and Emerging Therapies Assessment by DelveInsight | Ionis Pharmaceuticals, Inc., Agios Pharma, Silence Therapeutics, Phoenicia here
News-ID: 4185448 • Views: …
More Releases from ABNewswire

Windy City Chimney Heroes Announces Expanded Service Areas Across the Greater Ch …
Windy City Chimney Heroes, a long-standing chimney company established in 1997, has expanded its service areas across the Greater Chicago region. The company now serves Chicago, Evanston, Oak Park, Naperville, Schaumburg, Cicero, Skokie, Berwyn, and Arlington Heights. Owner Wesley Cook says the expansion will help meet rising demand for reliable chimney repair and inspection services.
Windy City Chimney Heroes, a trusted chimney repair and maintenance provider founded in 1997, has announced…

Injury 2 Wellness Centers Promotes Wellness Care Through Ongoing Chiropractic Su …
As the year draws to a close and many individuals begin setting health goals for the new year, Injury 2 Wellness Centers is encouraging Georgia residents to prioritize wellness care through consistent chiropractic treatment. Far beyond addressing back or neck pain, chiropractic care can serve as a foundation for long-term physical health, improved function, and overall quality of life.
Decatur, GA - December 10, 2025 - As the year draws to…

Avalon Tree Services Reminds Atlanta Homeowners to Prioritize Winter Tree Care f …
As temperatures drop and trees enter their dormant season, Avalon Tree Services is reminding homeowners across Greater Atlanta that winter is one of the most important times to invest in proper tree care. Cold weather, ice accumulation, and seasonal storms can all take a toll on tree health and stability-making preventative maintenance essential for safety and long-term growth.
Atlanta, GA - December 10, 2025 - As temperatures drop and trees enter…
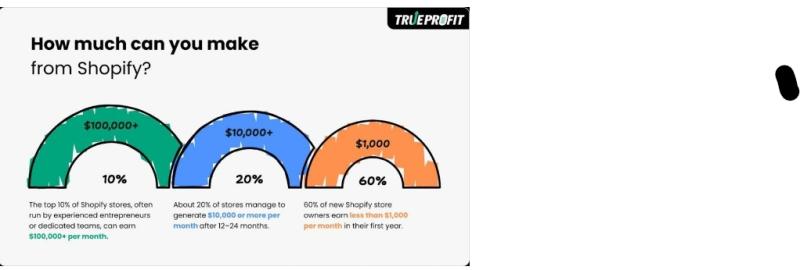
TrueProfit Shares 2026 Guidance for Shopify Startups to Focus on Profit First No …
As Shopify heads into 2026 with millions of active stores worldwide and continued growth in global ecommerce, TrueProfit - a Net Profit Analytics platform for Shopify merchants - is urging new founders to treat profitability as a starting requirement, not an afterthought.
With a low barrier to entry and a mature app ecosystem, Shopify remains one of the easiest ways to launch an online business. But TrueProfit's latest briefing on early-stage…
More Releases for Beta
Beta-lactam and Beta-lactamase Inhibitors Market Emerging Trends and Growth Pros …
The global beta-lactam and beta-lactamase inhibitors market is valued at approximately $60 billion in 2024, driven by the rising prevalence of bacterial infections and the increasing resistance to traditional antibiotics. The market is projected to reach around $85 billion by 2034, reflecting a robust growth trajectory. This results in a Compound Annual Growth Rate (CAGR) of approximately 6.5% during the forecast period of 2025-2034.
Exactitude Consultancy., Ltd. released a research report…
Beta-Lactams & Beta-Lactamase Inhibitors: A Persistent Powerhouse in the Antibio …
The antibiotics market, despite facing challenges, remains a critical sector in global healthcare. Within this landscape, the Beta-Lactam and Beta-Lactamase Inhibitor segment stands out as a high-opportunity area, driven by the continued prevalence of bacterial infections and the ongoing fight against antimicrobial resistance (AMR).
Market Dynamics and Growth Drivers
Beta-lactam antibiotics, including penicillins and cephalosporins, have been the cornerstone of antibacterial therapy for decades. Their broad spectrum of activity and generally favorable…
apuem - beta
Today the beta phase of apuem starts. Our goal is to create the largest web app collection, and for that we need your help. So if you know a useful online tool that you like to use, suggest it to us. See more: apuem.com/suggestions We appreciate any kind of participation. Thank you very much!
Apuem
Finkenhofstraße 27
60322 Frankfurt am Main
Germany
Jacob Kearson
support@apuem.com
More Information: apuem.com/contact
We and our community search for the best web apps…
Beta-lactam and Beta-lactamase Inhibitors Market to Reach $34,170 Million by 202 …
Global beta-lactam and beta-lactamase inhibitors market size was $27,126 million in 2018, and is projected to reach $34,170 million by 2028, growing at a CAGR of 2.3% from 2019 to 2028. The cephalosporin segment accounted more than two-fifths of the total beta-lactam and beta-lactamase inhibitors market share in 2018.
Download Report Sample PDF @ https://www.alliedmarketresearch.com/request-sample/5427
Beta-lactam and beta-lactamase inhibitors are chemical compounds of natural or semi-synthetic or synthetic origin. They inhibit…
Beta-lactam and Beta-lactamase Inhibitors Market Outlook from 2019 to 2028
"According to a new report published titled, ""India Heat Exchangers Market by Type, Material of Construction, and End-User Industry: Opportunity Analysis and Industry Forecast, 2019-2026,"" the India heat exchangers market accounted for revenue of $454.4 million in 2018, and is anticipated to generate $890.0 million by 2026. The market is projected to grow at a CAGR of 8.6% from 2019 to 2026.
Heat exchangers are devices that transfer energy between fluids…
Beta-lactam and Beta-lactamase Inhibitors Market Size, Share, Development by 202 …
Global Info Research offers a latest published report on Beta-lactam and Beta-lactamase Inhibitors Market Analysis and Forecast 2019-2025 delivering key insights and providing a competitive advantage to clients through a detailed report. This report focuses on the key global Beta-lactam and Beta-lactamase Inhibitors players, to define, describe and analyze the value, market share, market competition landscape, SWOT analysis and development plans in next few years.
To analyze the Beta-lactam and Beta-lactamase…
