Press release
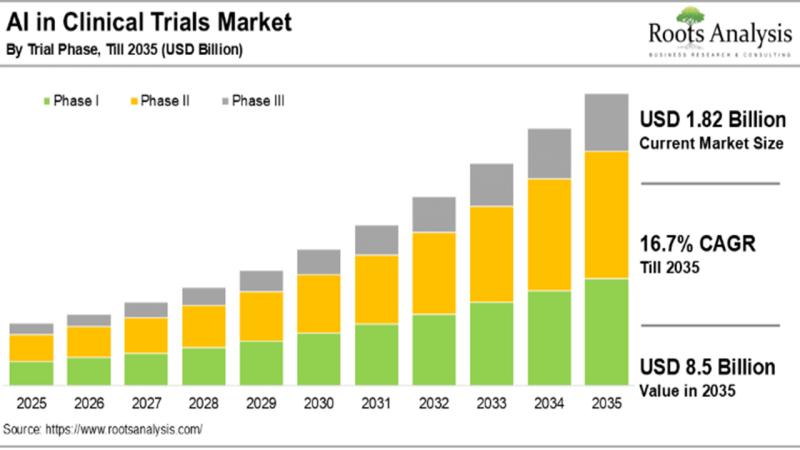
The AI in Clinical Trials Market is estimated to be worth USD 8.5 billion in 2035, predicts Roots Analysis
As a result of the excessive capital requirement and myriad of other complexities associated with clinical trials, pharmaceutical industry is increasingly relying on AI software and service providers to achieve desired outcomesRoots Analysis is pleased to announce the publication of its recent study, titled, "AI in Clinical Trials Market"
The report features an extensive study of the current market landscape, market size and future opportunities associated with the AI in clinical trials market, during the given forecast period. Further, the report highlights the efforts of several stakeholders engaged in this rapidly emerging segment of the pharmaceutical industry. Key takeaways of the AI in clinical trials market report includes:
1. An executive summary of the insights captured during our research.
2. A general overview of artificial intelligence in clinical trials, highlighting details on artificial intelligence and its subfields.
3. A detailed assessment of the current market landscape of the companies offering AI software and service for clinical trials, based on several relevant parameters.
4. Elaborate profiles of the prominent companies (shortlisted based on a proprietary criterion) developing AI software / AI solutions and offering services for clinical trials.
5. An insightful clinical trial analysis of completed / ongoing clinical trials leveraging AI, based on various relevant parameters.
6. A detailed analysis of the partnerships inked between stakeholders in the AI in clinical trials market, since 2018.
7. An analysis of the investments made, including seed financing, venture capital financing, capital raised from IPOs, grants, debt financing and other equity, and subsequent offerings.
8. A detailed analysis of the initiatives taken by big pharma players related to AI in clinical trials, based on various relevant parameters.
9. An insightful framework depicting the implementation of several advanced tools and technologies, such as blockchain, big data analytics, real-world evidence, digital twins, cloud computing and internet of things (IoT) at different steps of a clinical study.
10. A detailed cost saving analysis, highlighting the overall cost saving potential of AI in clinical trials till 2035.
To request quote of this report, please visit:
https://www.rootsanalysis.com/reports/ai-based-clinical-trial-solutions/request-quote.html
11. A detailed market forecast, featuring analysis of the current and projected future opportunity across key market segments (listed below)
Trial Phase
a) Phase I
b) Phase II
c) Phase III
Target Therapeutic Area
a) Oncological Disorders
b) Cardiovascular Disorders
c) CNS Disorders
d) Infectious Diseases
e) Metabolic Disorders
f) Other Disorders
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/ai-based-clinical-trial-solutions/request-sample.html
End-User
a) Biotechnology and Pharmaceutical Companies
b) Academic Research Institute and Other end-users
Key Geographical Region
a) North America
b) Europe
c) Asia-Pacific
d) Middle East and North Africa
e) Latin America
Key companies covered in the report
a) AiCure
b) Antidote Technologies
c) Deep 6 AI
d) Innoplexus
e) IQVIA
f) Median Technologies
g) Medidata
h) Mendel.ai
i) Phesi
j) Saama Technologies
k) Signant Health
l) Trials.ai
Browse Full Report Description + Research Methodology + Table of Content + Infographics here:
https://www.rootsanalysis.com/reports/ai-based-clinical-trial-solutions.html
Gaurav Chaudhary
Email: Gaurav.chaudhary@rootsanalysis.com or sales@rootsanalysis.com
Website: https://www.rootsanalysis.com
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights. All reports provided by us are structured in a way that enables the reader to develop a thorough perspective on the given subject. Apart from writing reports on identified areas, we provide bespoke research / consulting services dedicated to serve our clients in the best possible way.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The AI in Clinical Trials Market is estimated to be worth USD 8.5 billion in 2035, predicts Roots Analysis here
News-ID: 4110341 • Views: …
More Releases from Roots Analysis
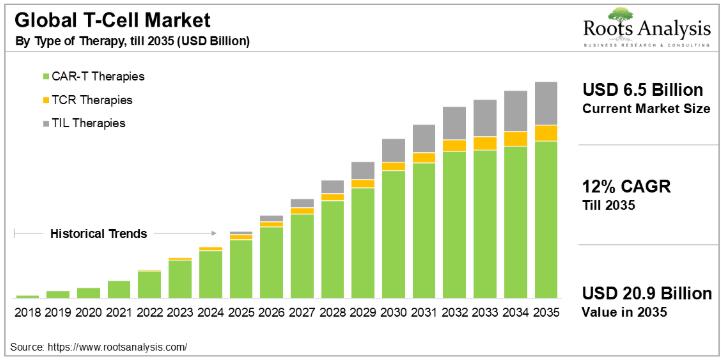
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
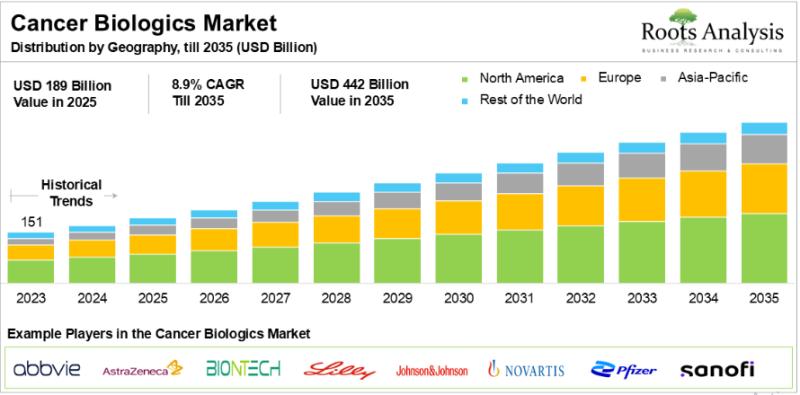
Cancer Biologics Market: Unmet Need and Treatment Guidelines
Owing to the increasing mortality rates and growing need for novel modalities to treat oncological disorders, several researchers and industry stakeholders have shifted their focus on the development of safe and effective biologic therapies. Cancer biologics are the class of therapeutic agents, which primarily modulate immune responses or directly inhibits oncogenic pathways in malignancies. These therapies, such as monoclonal antibodies, specifically target tumor-activating genes, facilitate antibody-dependent cellular cytotoxicity and complement…
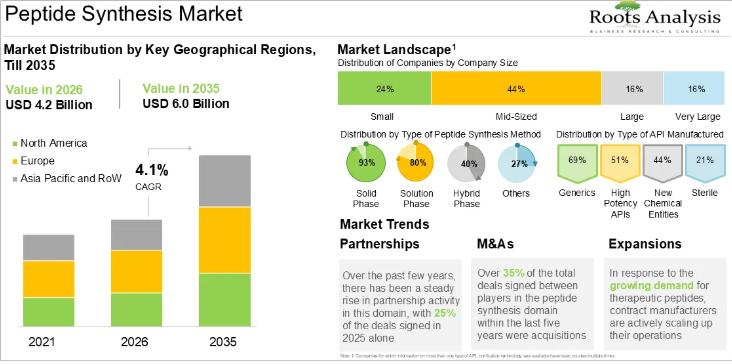
Peptide Synthesis: Supporting Next Generation Targeted Therapies
Peptides are specialized molecules composed of short chains of amino acids and are used primarily as the active ingredients in a new class of targeted therapeutics. Peptide synthesis is achieved through various advanced methodologies, including chemical processes like solid-phase (SPPS) and liquid-phase (LPPS), as well as hybrid and non-chemical approaches.
Peptides are being extensively used as therapeutics to treat various disorders, including metabolic diseases, oncological disorders, and hormonal imbalances due to…
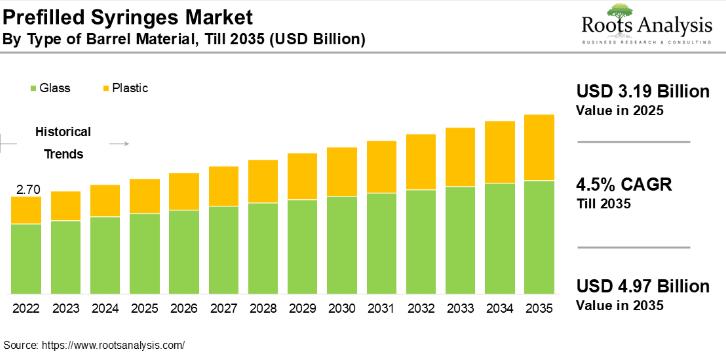
Prefilled Syringes Market Size to Hit USD 4.97 billion by 2035| Exclusive Report …
Prefilled syringes: setting new standards for safe and efficient drug administration. With the increasing population, the incidence rates of various chronic diseases, such as diabetes, autoimmune disorders, cardiovascular disorders and oncological disorders, are witnessing an upward trend.
The global prefilled syringes market, valued at USD 3.02 billion in 2024, is project to reach USD 3.19 billion in 2025 and USD 4.97 billion by 2035, representing a CAGR of 4.5% during the…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…