Press release
Rising Incidence Of Cardiovascular Diseases Fuels Growth Of The Cardiovascular Clinical Trials Market Driver: Leading Transformation in the Cardiovascular Clinical Trials Market in 2025
What combination of drivers is leading to accelerated growth in the cardiovascular clinical trials market?The upward trend in cardiovascular diseases is predicted to propel the cardiovascular clinical trials market's expansion. Cardiovascular disease is a term that encompasses a range of conditions impacting the heart and blood vessels including coronary artery disease, heart failure, and stroke. A rise in cardiovascular diseases can be attributed to multiple factors like inactive lifestyles, poor diet, tobacco use, obesity, hypertension, diabetes, and hereditary influences. Cardiovascular clinical trials play a crucial role in identifying and confirming new medications that can better control risk factors such as high blood pressure, high cholesterol, and diabetes - major factors contributing to cardiovascular diseases. To illustrate, the Minnesota Department of Health, a US-based state health institution, disclosed in September 2024 that around 30% of Minnesota's adult population, nearly 1.4 million individuals, reported high blood pressure in 2023, and in 2022, diseases caused by hypertension were the primary or secondary cause of death for about 14,225 residents, accounting for almost 28% of the total fatalities in the state. Therefore, the surging incidence of cardiovascular diseases is fueling the growth of the cardiovascular clinical trials market.
Get Your Cardiovascular Clinical Trials Market Report Here:
https://www.thebusinessresearchcompany.com/report/cardiovascular-clinical-trials-global-market-report
What is the projected compound annual growth rate (CAGR) of the cardiovascular clinical trials market from 2025 to 2034, and what factors influence it?
In the past few years, the cardiovascular clinical trials market has seen substantial growth. The market is projected to increase from $5.34 billion in 2024 to $5.73 billion in 2025, growing at a compound annual growth rate (CAGR) of 7.4%. The historic growth of this market is due to factors such as a rise in myocardial infarction, evolving regulatory landscape, increase in disease burden, progress in genomics, and a heightened demand for new drug development.
In the near future, a robust expansion is predicted for the cardiovascular clinical trials market, with its value set to hit $7.53 billion by 2029. This increase, representing a compound annual growth rate (CAGR) of 7.0%, is mainly driven by factors such as an aging global population, the rise of emerging markets, the prevalence of precision medicine, advances in digital health technologies, and a shift towards patient-centered trials. The period forecasted also sees trends like heightened collaboration, technological progress, innovation in products, development of new products, and the introduction of generic versions of combination drugs.
Get Your Free Sample Now - Explore Exclusive Market Insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15532&type=smp
How are the latest trends influencing the growth of the cardiovascular clinical trials market?
Leading corporations in the cardiovascular clinical trials market are increasingly leveraging strategic alliances like contract research organization (CRO) partnerships to broaden their outreach and attract a larger customer segment. These CRO partnerships, pivotal in the clinical research sector, entail cooperation across different companies to offer unique services and assistance in varied dimensions of clinical trials and research. For example, in September 2023, Cereno Scientific AB, a biopharmaceutical firm based in Sweden, entered a strategic collaboration with Clinical Trial Consultants (CTC), a leading full-service CRO in Sweden specializing in clinical conduct, for carrying out a Phase I study on CS014, a histone deacetylase inhibitor designed to thwart arterial and venous thrombosis. Apart from this, CTC will also contribute to the Phase I preparation processes which include creating the study protocol and managing the clinical trial application process for conducting the study in Sweden. The first-ever Phase I trial is planned to commence in the first half of 2024. This partnership heralds a monumental step forward in cardiovascular health research.
What are the major segments of the cardiovascular clinical trials market and their role in driving growth?
The cardiovascular clinical trials market covered in this report is segmented -
1) By Phase: Phase I, Phase II, Phase III, Phase IV
2) By Study Design: Interventional, Observational, Expanded Access
3) By Indication: Acute Coronary Syndrome, Coronary Artery Disease, Ischemic Heart Disease, Pulmonary Arterial Hypertension, Stroke, Cardiac Arrhythmias, Heart Failure, Other Indications
Subsegments:
1) By Phase I: First-in-Human Trials, Dose Escalation Studies, Safety and Tolerability Studies, Pharmacokinetics And Pharmacodynamics Studies
2) By Phase II: Efficacy Studies, Optimal Dosage And Administration Route Studies, Early Safety And Efficacy Trials, Biomarker Development Trials
3) By Phase III: Large-Scale Efficacy Trials, Randomized Controlled Trials (RCTs), Long-Term Safety And Efficacy Studies, Multicenter Trials
4) By Phase IV: Post-Marketing Surveillance, Long-Term Safety Studies, Real-World Evidence (RWE) Studies, Comparative Effectiveness Research
Unlock Exclusive Market Insights - Purchase Your Research Report Now!
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=15532
North America was the largest region in the cardiovascular clinical trials market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the cardiovascular clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Who are the key firms paving the way for growth in the cardiovascular clinical trials market?
Major companies operating in the cardiovascular clinical trials market are Pfizer Inc., Johnson & Johnson, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., AstraZeneca PLC, Novartis AG, Eli Lilly and Company, Gilead Sciences Inc., Amgen Inc., Boehringer Ingelheim International GmbH, Merck & Co. Inc., Baxter International Inc., IQVIA Holdings Inc., SGS S.A., PPD Inc., WuXi AppTec Co. Ltd., Caidya, Syneos Health Inc., Charles River Laboratories International Inc., Sanofi, ICON plc, Medpace Holdings Inc., Cardiovascular Clinical Sciences., ProRelix Services LLP, Worldwide Clinical Trials
Customize Your Report - Get Tailored Market Insights!
https://www.thebusinessresearchcompany.com/customise?id=15532&type=smp
What Is Covered In The Cardiovascular Clinical Trials Global Market Report?
•Market Size Forecast: Examine the cardiovascular clinical trials market size across key regions, countries, product categories, and applications.
•Segmentation Insights: Identify and classify subsegments within the cardiovascular clinical trials market for a structured understanding.
•Key Players Overview: Analyze major players in the cardiovascular clinical trials market, including their market value, share, and competitive positioning.
•Growth Trends Exploration: Assess individual growth patterns and future opportunities in the cardiovascular clinical trials market.
•Segment Contributions: Evaluate how different segments drive overall growth in the cardiovascular clinical trials market.
•Growth Factors: Highlight key drivers and opportunities influencing the expansion of the cardiovascular clinical trials market.
•Industry Challenges: Identify potential risks and obstacles affecting the cardiovascular clinical trials market.
•Competitive Landscape: Review strategic developments in the cardiovascular clinical trials market, including expansions, agreements, and new product launches.
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Europe: +44 207 1930 708,
Asia: +91 88972 63534,
Americas: +1 315 623 0293 or
Email: mailto:info@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rising Incidence Of Cardiovascular Diseases Fuels Growth Of The Cardiovascular Clinical Trials Market Driver: Leading Transformation in the Cardiovascular Clinical Trials Market in 2025 here
News-ID: 3921353 • Views: …
More Releases from The Business Research Company
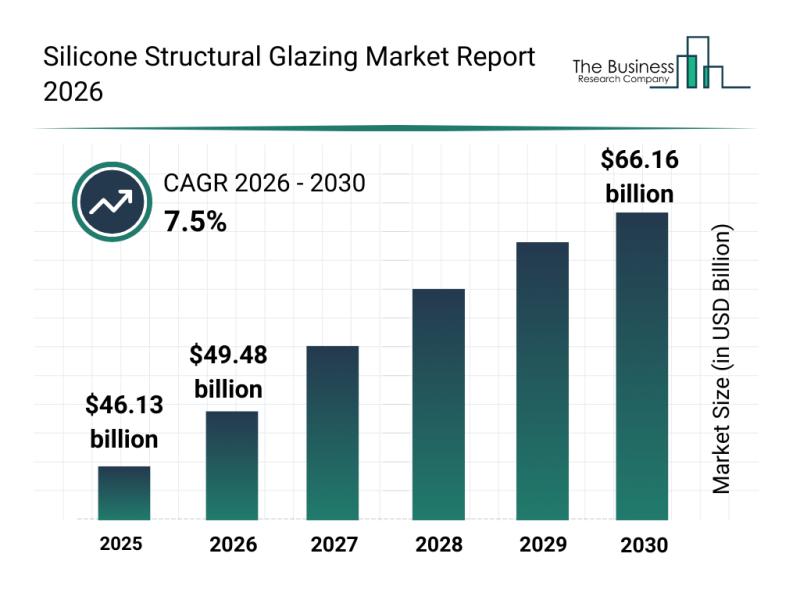
Leading Companies Solidify Their Presence in the Silicone Structural Glazing Mar …
The silicone structural glazing market is positioned for significant expansion in the coming years, driven by advances in building technology and increased environmental awareness. This sector is evolving rapidly as demand grows for more energy-efficient and aesthetically appealing architectural solutions. Let's explore the market's current size, key players, emerging trends, and the main segments that are shaping its future.
Silicone Structural Glazing Market Value Forecast Through 2030
The market for silicone…
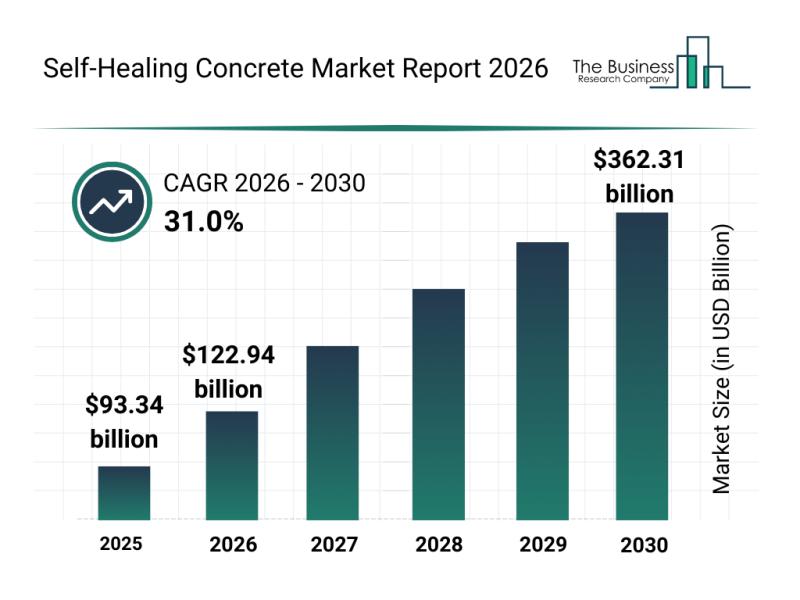
Future Prospects: Key Trends Shaping the Self-Healing Concrete Market up to 2030
The self-healing concrete market is capturing significant attention as innovations and sustainability demands rise in construction. This sector is set to experience remarkable growth due to advancements in materials and technology, shaping the future of durable and intelligent infrastructure solutions. Let's explore the market's size, key players, emerging trends, and segment outlook to understand its trajectory.
Projected Market Size and Growth Prospects for the Self-Healing Concrete Market
The self-healing concrete market…
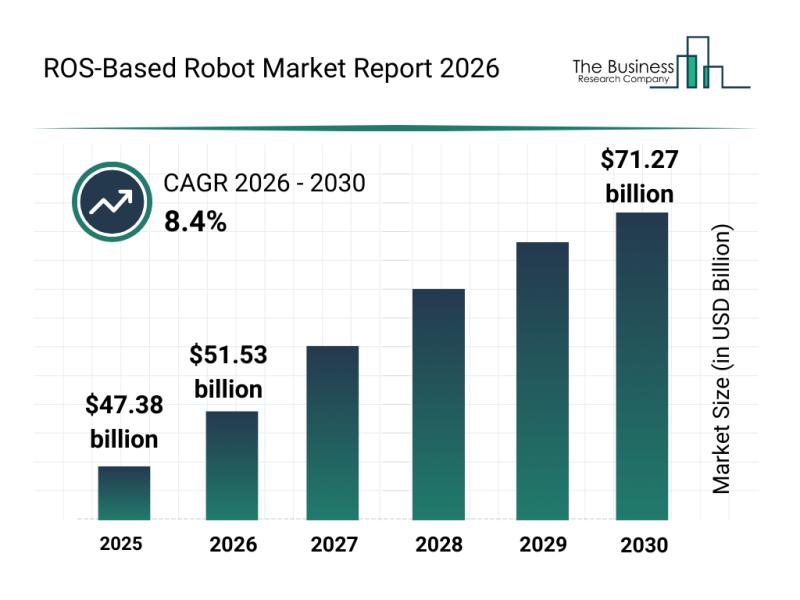
Analysis of Key Market Segments Driving the ROS-Based Robot Industry
The ROS-based robot market is positioned for substantial growth as robotics technology continues to advance rapidly. With increasing innovation in software, hardware, and AI integration, this sector is set to transform multiple industries by 2030. Below, we explore the market's future size, leading companies, key trends, and segmentation details to understand its evolving landscape.
Projected Market Size and Expansion of the ROS-Based Robot Market
The ROS-based robot market is anticipated to…
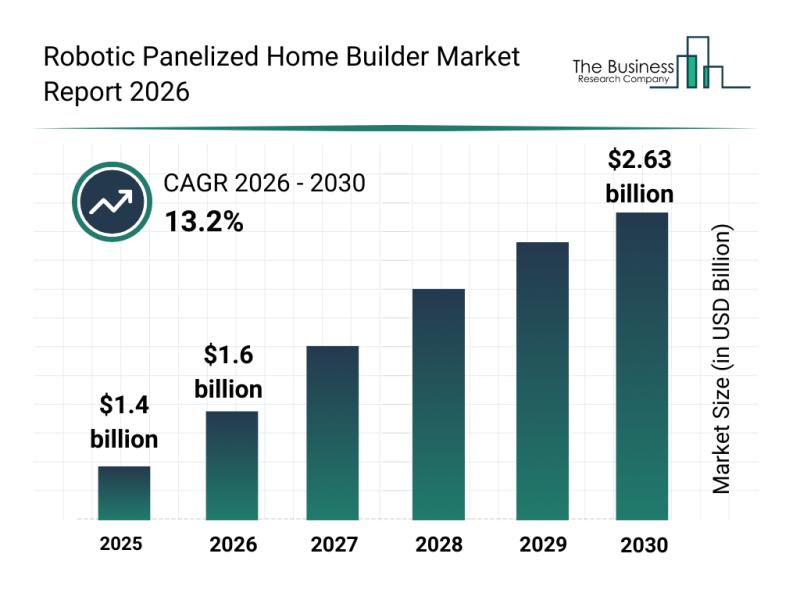
Global Trends Overview: The Rapid Evolution of the Robotic Panelized Home Builde …
The robotic panelized home builder market is positioned for impressive growth in the coming years as automation and robotics increasingly transform construction processes. Driven by technological advancements and expanding prefab housing projects, this market is set to reshape how homes are built with greater speed and efficiency. Let's explore the market's size, leading companies, emerging trends, and key segments that are shaping its future.
Strong Growth Forecast for the Robotic Panelized…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
