Press release
Lewy Body Dementia Pipeline 2025: FDA Approvals and Clinical Trials Landscape with MOA and ROA Highlights by DelveInsight | Jazz Pharma, Allergan, Noven Pharma, Takeda Pharma, Eli Lilly, Mallinckrodt Pharma
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Lewy Body Dementia pipeline constitutes key companies continuously working towards developing Lewy Body Dementia treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.The Lewy Body Dementia Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers acquisition, funding, designations, and other product-related details.
"Lewy Body Dementia Pipeline Insight, 2025" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Lewy Body Dementia Market.
Some of the key takeaways from the Lewy Body Dementia Pipeline Report: https://www.delveinsight.com/sample-request/lewy-body-dementia-lbd-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
• Companies across the globe are diligently working toward developing novel Lewy Body Dementia treatment therapies with a considerable amount of success over the years.
• Lewy Body Dementia companies working in the treatment market are Eisai, EIP Pharma, Sun Pharma, Axovant Sciences Ltd., Cognition Therapeutics, Cephalon, Athira Pharma, Ortho-McNeil Neurologics, Inc., H. Lundbeck A/S, Axovant Sciences Ltd., GE Healthcare, Aptinyx, NeuroActiva, Inc., CuraSen Therapeutics, Inc., ACADIA Pharmaceuticals Inc, and others, are developing therapies for the Lewy Body Dementia treatment
• Emerging Lewy Body Dementia therapies in the different phases of clinical trials are- E2027, Neflamapimod, K0706, RVT-101, CT1812, K0706, Irsenontrine, Donepezil, ATH-1017, Galantamine, Memantine, Nelotanserin, DatSCAN, NYX-458, N-831(Traneurocin), CST-103, CST-107, Pimavanserin, and others are expected to have a significant impact on the Lewy Body Dementia market in the coming years.
• In December 2024, Cognition Therapeutics, Inc. (NASDAQ: CGTX), a clinical-stage biopharmaceutical company focused on developing treatments for neurodegenerative diseases, announced topline results from the exploratory Phase 2 'SHIMMER' study. The findings revealed that CT1812 showed significant therapeutic benefits across behavioral, functional, cognitive, and motor assessments in patients with dementia with Lewy bodies (DLB).
• In April 2024, Cognition Therapeutics, a US-based biopharmaceutical firm, completed subject enrollment for its Phase II SHIMMER study of CT1812, aimed at treating mild-to-moderate dementia with Lewy bodies (DLB). This study assesses the safety and efficacy of CT1812, a novel treatment for adults with DLB. The double-blind, placebo-controlled trial has enrolled 120 adults with mild-to-moderate DLB, who will receive either a placebo or one of two oral doses of CT1812 over six months.
• In February 2024, CervoMed Inc. (NASDAQ: CRVO), a clinical-stage company dedicated to developing therapies for degenerative brain diseases, announced that data from the AscenD-LB Phase 2a trial on neflamapimod treatment in patients with dementia with Lewy bodies (DLB) have been published online in the Journal of Prevention of Alzheimer's Disease (JPAD).
• In August 2023, EIP Pharma, a US-based biopharmaceutical company, has administered the first dose in its RewinD-LB clinical trial, investigating neflamapimod for treating dementia with Lewy bodies (DLB). This Phase IIb trial (NCT05869669) of the pipeline drug neflamapimod (VX-745) follows encouraging results from the earlier Phase IIa AscenD-LB trial.
Lewy Body Dementia Overview
Lewy Body Dementia (LBD) is a progressive brain disorder characterized by abnormal deposits of a protein called alpha-synuclein in the brain. These deposits, known as Lewy bodies, affect chemicals in the brain and can lead to problems with thinking, movement, behavior, and mood. LBD encompasses two related conditions: Dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD).
Get a Free Sample PDF Report to know more about Lewy Body Dementia Pipeline Therapeutic Assessment-
https://www.delveinsight.com/report-store/lewy-body-dementia-lbd-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Emerging Lewy Body Dementia Drugs Under Different Phases of Clinical Development Include:
• E2027: Eisai
• Neflamapimod: EIP Pharma
• K0706: Sun Pharma
• RVT-101: Axovant Sciences Ltd.
• CT1812: Cognition Therapeutics
• K0706: Cephalon
• Irsenontrine: Eisai Inc.
• Neflamapimod: EIP Pharma Inc
• Donepezil: Eisai Co., Ltd.
• ATH-1017: Athira Pharma
• Galantamine: Ortho-McNeil Neurologics, Inc.
• Memantine: H. Lundbeck A/S
• Nelotanserin: Axovant Sciences Ltd.
• DatSCAN: GE Healthcare
• NYX-458: Aptinyx
• N-831(Traneurocin): NeuroActiva, Inc.
• CST-103, CST-107: CuraSen Therapeutics, Inc.
• Pimavanserin: ACADIA Pharmaceuticals Inc.
Lewy Body Dementia Pipeline Therapeutics Assessment
• Lewy Body Dementia Assessment by Product Type
• Lewy Body Dementia By Stage and Product Type
• Lewy Body Dementia Assessment by Route of Administration
• Lewy Body Dementia By Stage and Route of Administration
• Lewy Body Dementia Assessment by Molecule Type
• Lewy Body Dementia by Stage and Molecule Type
DelveInsight's Lewy Body Dementia Report covers around products under different phases of clinical development like
• Late-stage products (Phase III)
• Mid-stage products (Phase II)
• Early-stage product (Phase I)
• Pre-clinical and Discovery stage candidates
• Discontinued & Inactive candidates
• Route of Administration
Further Lewy Body Dementia product details are provided in the report. Download the Lewy Body Dementia pipeline report to learn more about the emerging Lewy Body Dementia therapies
https://www.delveinsight.com/sample-request/lewy-body-dementia-lbd-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Some of the key companies in the Lewy Body Dementia Therapeutics Market include:
Key companies developing therapies for Lewy Body Dementia are - Jazz Pharmaceuticals Inc., Allergan Plc., Noven Pharmaceuticals Inc., Takeda Pharmaceutical Company Limited, Eli Lilly and Company, Mallinckrodt Pharmaceuticals, BioArctic AB, Sumitomo Pharma Co. Ltd., Novartis AG, GSK Plc., and others.
Lewy Body Dementia Pipeline Analysis:
The Lewy Body Dementia pipeline report provides insights into
• The report provides detailed insights about companies that are developing therapies for the treatment of Lewy Body Dementia with aggregate therapies developed by each company for the same.
• It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Lewy Body Dementia Treatment.
• Lewy Body Dementia key companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
• Lewy Body Dementia Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
• Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Lewy Body Dementia market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Download Sample PDF Report to know more about Lewy Body Dementia drugs and therapies
https://www.delveinsight.com/sample-request/lewy-body-dementia-lbd-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Lewy Body Dementia Pipeline Market Strengths
• Increasing research and development to understand the diversity of the disease might improve the diagnosis of LBD thereby resulting in a lucrative market opportunity.
• Current treatments are effective in relieving the symptoms and improving the quality of life of the patients
Lewy Body Dementia Pipeline Market Opportunities
• Several organizations are actively working to provide information and increase awareness of such disorders.
• There is only one approved treatment option for LBD which opens a platform of new therapies to boost the market ofLBD.
• The pipeline for PMM is narrow as there is limited R&D activity
Scope of Lewy Body Dementia Pipeline Drug Insight
• Coverage: Global
• Key Lewy Body Dementia Companies: Eisai, EIP Pharma, Sun Pharma, Axovant Sciences Ltd., Cognition Therapeutics, Cephalon, Athira Pharma, Ortho-McNeil Neurologics, Inc., H. Lundbeck A/S, Axovant Sciences Ltd., GE Healthcare, Aptinyx, NeuroActiva, Inc., CuraSen Therapeutics, Inc., ACADIA Pharmaceuticals Inc, and others
• Key Lewy Body Dementia Therapies: E2027, Neflamapimod, K0706, RVT-101, CT1812, K0706, Irsenontrine, Donepezil, ATH-1017, Galantamine, Memantine, Nelotanserin, DatSCAN, NYX-458, N-831(Traneurocin), CST-103, CST-107, Pimavanserin, and others
• Lewy Body Dementia Therapeutic Assessment: Lewy Body Dementia current marketed and Lewy Body Dementia emerging therapies
• Lewy Body Dementia Market Dynamics: Lewy Body Dementia market drivers and Lewy Body Dementia market barriers
Request for Sample PDF Report for Lewy Body Dementia Pipeline Assessment and clinical trials
https://www.delveinsight.com/sample-request/lewy-body-dementia-lbd-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Table of Contents
1. Lewy Body Dementia Report Introduction
2. Lewy Body Dementia Executive Summary
3. Lewy Body Dementia Overview
4. Lewy Body Dementia- Analytical Perspective In-depth Commercial Assessment
5. Lewy Body Dementia Pipeline Therapeutics
6. Lewy Body Dementia Late Stage Products (Phase II/III)
7. Lewy Body Dementia Mid Stage Products (Phase II)
8. Lewy Body Dementia Early Stage Products (Phase I)
9. Lewy Body Dementia Preclinical Stage Products
10. Lewy Body Dementia Therapeutics Assessment
11. Lewy Body Dementia Inactive Products
12. Company-University Collaborations (Licensing/Partnering) Analysis
13. Lewy Body Dementia Key Companies
14. Lewy Body Dementia Key Products
15. Lewy Body Dementia Unmet Needs
16 . Lewy Body Dementia Market Drivers and Barriers
17. Lewy Body Dementia Future Perspectives and Conclusion
18. Lewy Body Dementia Analyst Views
19. Appendix
20. About DelveInsight
Related Reports:
Lewy Body Dementia Market https://www.delveinsight.com/report-store/lewy-body-dementia-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
DelveInsight's 'Lewy Body Dementia Market Insights, Epidemiology, and Market Forecast-2034' report delivers an in-depth understanding of the 7MM, historical and forecasted epidemiology as well as the 7MM market trends in the United States, EU5 (Germany, France, Italy, Spain, and United Kingdom),
Latest Reports:
• Cardiac Arrythmia Market: https://www.delveinsight.com/report-store/ambulatory-arrhythmia-monitoring-devices-market
• Alopecia Market: https://www.delveinsight.com/report-store/alopecia-areata-market
• Optical Coherence Tomography Devices Market: https://www.delveinsight.com/report-store/optical-coherence-tomography-oct-devices-market
• Prediabetes Market: https://www.delveinsight.com/report-store/prediabetes-market
• Scoliosis Market: https://www.delveinsight.com/report-store/scoliosis-market
• Psoriasis Market: https://www.delveinsight.com/report-store/psoriasis-market
• Tendinitis Market: https://www.delveinsight.com/report-store/tendonitis-market
• Vascular Closure Devices Market: https://www.delveinsight.com/report-store/vascular-closure-devices-market
Contact Us:
Gaurav Bora
gbora@delveinsight.com
+14699457679
Healthcare Consulting
https://www.delveinsight.com/consulting-services
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate business growth and overcome challenges with a practical approach.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Lewy Body Dementia Pipeline 2025: FDA Approvals and Clinical Trials Landscape with MOA and ROA Highlights by DelveInsight | Jazz Pharma, Allergan, Noven Pharma, Takeda Pharma, Eli Lilly, Mallinckrodt Pharma here
News-ID: 3867783 • Views: …
More Releases from DelveInsight Business Research
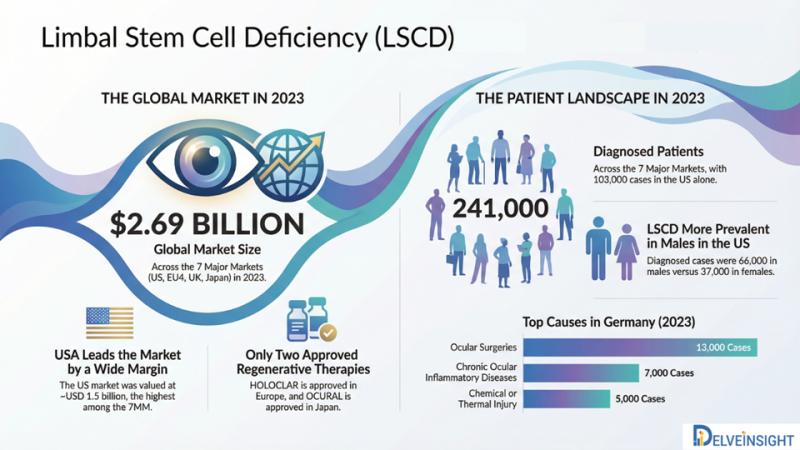
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
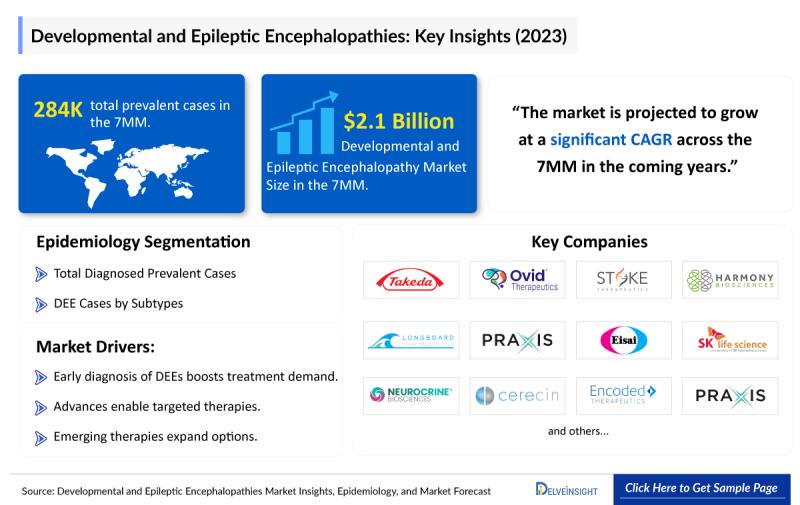
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerat …
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.
The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs…
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
More Releases for Lewy
Lewy Body Dementia (LBD) Market to Reach USD 5.12 Billion by 2034
Pune, India - December 2025 - The global Lewy Body Dementia (LBD) Market, valued at USD 2.98 billion in 2024, is projected to reach USD 5.12 billion by 2034, growing at a 5.6% CAGR (2025-2034), according to Exactitude Consultancy. Rising neurodegenerative disease prevalence, improved diagnostic imaging, and growing awareness of cognitive and behavioral symptoms are driving market expansion.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71909
Market Summary
The Lewy Body…
Lewy Body Dementia Treatment Market Trends That Will Shape the Next Decade: Insi …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Lewy Body Dementia Treatment Market Size By 2025?
The market for lewy body dementia therapies has experienced robust expansion lately, projected to increase its valuation from $4.43 billion in 2024 to $4.69 billion in 2025, reflecting a compound annual growth rate (CAGR) of 5.8%; this…
Lewy Body Dementia Market to Reach USD 9.4 Billion by 2034
Lewy Body Dementia (LBD) is the second most common form of progressive dementia after Alzheimer's disease, accounting for 10-15% of dementia cases. It is caused by the abnormal accumulation of alpha-synuclein protein (Lewy bodies) in the brain, leading to cognitive decline, visual hallucinations, fluctuating alertness, REM sleep behavior disorder, and Parkinsonism symptoms.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71909
With aging populations and rising awareness, the LBD market is…
Lewy Body Dementia Market Forecast 2025-2034: Comprehensive Analysis And Growth …
We've updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
What Is the Projected Growth of the Lewy Body Dementia Market?
In recent times, the market size for Lewy body dementia has witnessed substantial growth. It is projected to increase from $1.12 billion in 2024 to $1.21 billion in 2025, with a compound annual growth rate (CAGR) of 7.5%. The historic growth…
Lewy Body Dementia Treatment Market Challenges: Growth, Share, Value, Trends and …
Lewy Body Dementia Treatment Market Size And Forecast by 2031
The LBD Therapy Market is expanding rapidly, driven by increasing consumer demand, technological advancements, and industry-wide innovation. According to top market research firms, businesses in the Neurodegenerative Disorder Treatment Market are prioritizing digital transformation, product development, and data-driven decision-making to stay competitive. With rising investments in automation and efficiency, the Dementia Care Market is evolving to meet changing customer preferences.…
Lewy Body Dementia Treatment Market Focuses on Advancements in Diagnosis and The …
Global Lewy Body Dementia Treatment Market, By Drug Type (Cholinesterase Inhibitors, Antipsychotic Drugs, Antidepressants, Benzodiazepine, and Modafinil), Application Type (Parkinson's Disease and Alzheimer's Disease), Mode of Purchase (Prescription and Over the Counter), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies and Others) - Industry Trends and Forecast to 2031.
Data Bridge Market Research analyses that the Global Lewy Body Dementia Treatment Market which was USD 5.66 Billion in 2023…
