Press release
Regulatory T-cells (Tregs) Therapies Market Key Players Analysis - Abata Therapeutics (US), Cellenkos Inc (US), Coya Therapeutics (US), Roche (Switzerland), Caladrius Biosciences (US).
Regulatory T-cells (Tregs) Therapies Market - Future Pipeline/Clinical Trial Analysis and Revenue Forecast SnapshotInsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Regulatory T-cells (Tregs) therapies Market Trends, Clinical Trial/Pipeline Analysis, Funding Analysis, Industry Competition Analysis, Revenue and Forecast To 2030."
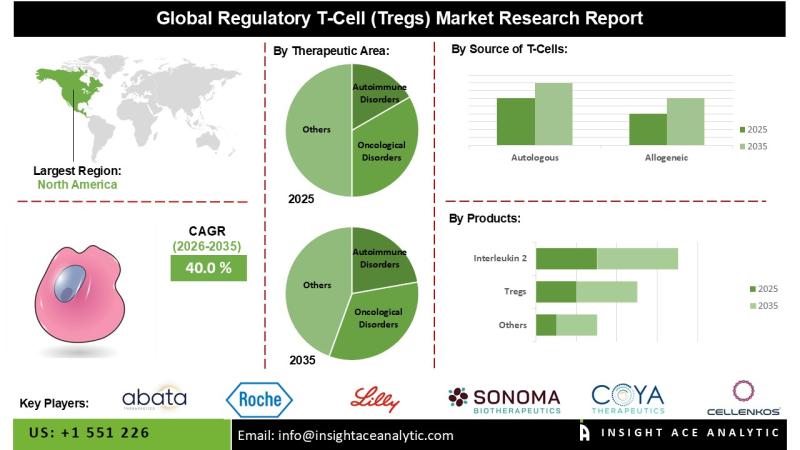
According to the most recent research report, the global regulatory T-cells (Tregs) therapeutics market is estimated to reach $1,138.6 million by 2030, growing at a CAGR of 52.8% from 2025 to 2030. There is currently no approved marketed product for Regulatory T-cells (Tregs) therapies in the global market, with the first product likely to be launched in 2024-2025.
Get Free Access to Demo Report, Excel Pivot and ToC :https://www.insightaceanalytic.com/request-sample/1200
Regulatory T cells (Tregs) are a group of immune cells that can decrease the immune response and contribute to homeostasis and self-tolerance. Many preclinical investigations have indicated that Tregs can suppress T cell proliferation, cytokine generation, and play an important role in modulating autoimmune reactions. The regulatory T-cells (Tregs) therapies market is growing due to a variety of factors, including the high prevalence of autoimmune and inflammatory diseases, the growing geriatric population, increased public awareness of healthcare and wellness, the emergence of new techniques such as next-generation T-cell immunotherapy, rising healthcare expenditure, and increased R&D investments to innovate adoptive Treg therapies.
The current COVID-19 pandemic has prompted researchers to focus more on understanding the novel applications of T-cell treatments in viral infection research. In December 2020, Miltenyi Biotec (Germany) introduced new kits for efficiently stimulating and analyzing SARS-CoV-2-reactive T cells. The kits, designed for quick detection in PBMC samples following stimulation with the SARS-CoV-2 PepTivator Peptide Pools published earlier this year, represent decades of experience in designing solutions for virus-specific T cell research. As a result, increased R&D activities are expected to open up new potential for the regulatory T-cell (Treg) therapeutics market in the coming years.
However, the difficult manufacture of regulatory T-cell immunotherapies, the high cost of Treg therapies, and the limited adverse effects of these therapies are expected to impede market acceptance in the coming years.
The Asia Pacific region is expected to grow significantly during the forecast period (2025-2030) as a result of the government's increased research funding and investments, rising chronic disease prevalence, and rapid adoption of advanced drug development and therapy techniques.
Expert Knowledge, Just a Click Away:https://calendly.com/insightaceanalytic/30min?month=2024-02
Major market players operating in the regulatory T-cells (Tregs) therapies market include Abata Therapeutics (US), Cellenkos Inc (US), Coya Therapeutics (US), Roche (Switzerland), Caladrius Biosciences (US), Sonoma Biotherapeutics (US), Nektar Therapeutics (US), Eli Lilly and Company (US), REGiMMUNE (US), Miltenyi Biotec (Germany), TeraImmune (US), TRACT Therapeutics (US), VT Bio (South Korea), Amgen (US), Sangamo Therapeutics (TxCell) (US), Pfizer Inc. (US), PolTREG S.A.. (Poland), Parvus Therapeutics (Canada), ILTOO Pharma (France), Philogen S.p.A. (Italy), Celgene (US), AHEAD THERAPEUTICS S.L. (Spain) among others.
Key developments in the market:
• In July 2021, the U.S. FDA granted Orphan Drug Designation to Coya Therapeutics's ALS001, an autologous, expanded Treg cell therapy in development to treat amyotrophic lateral sclerosis (ALS).
• In February 2021, Coya Therapeutics, Inc. (US) merged with Nicoya Health, Inc. It raised $10 million in Series A financing from institutional and accredited investors to advance the pipeline of regulatory T cell therapeutics optimized for neurodegeneration and autoimmune diseases.
• In May 2021, Nektar Therapeutics (US) announced the first publication of NKTR-358, a first-in-class composition of stable PEG conjugates of native IL-2 designed to selectively stimulate expansion and selective function of T regulatory cells, in the journal of translational autoimmunity. NKTR-358 is in the development for the treatment of a range of autoimmune and inflammatory disorders.
• In November 2020, the U.S FDA approved TRACT Therapeutics's investigational drug candidate, TRK-001, for its potential to reduce organ rejection following solid organ transplantation.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/1200
Market Segments
Regulatory T-cells (Tregs) therapies Market, by Target Indication, 2025-2030 (Valu US$ Mn)
• Crohn Disease
• Bipolar Disorder
• Allergic Rhinoconjunctivitis
• COVID 19
• Diabetes Mellitus
• Systemic Lupus Erythematosus
• Alzheimer Disease
• Graft Vs Host Disease
Regulatory T-cells (Tregs) therapies Market, by Products, 2025-2030 (Valu US$ Mn)
• Tregs
• Interleukin 2
• Monoclonal Antibodies
• Small Molecules
• Other Products
Regulatory T-cells (Tregs) therapies Market, by Region, 2025-2030 (Value US$ Mn)
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
North America Regulatory T-cells (Tregs) therapies Market, by Country, 2025-2030 (Value US$ Mn)
• U.S.
• Canada
Europe Regulatory T-cells (Tregs) therapies Market, by Country, 2025-2030 (Value US$ Mn)
• Germany
• France
• Italy
• Spain
• Russia
• Rest of Europe
Asia Pacific Regulatory T-cells (Tregs) therapies Market, by Country, 2025-2030 (Value US$ Mn)
• India
• China
• Japan
• South Korea
• Australia & New Zealand
Latin America Regulatory T-cells (Tregs) therapies Market, by Country, 2025-2030 (Value US$ Mn)
• Brazil
• Mexico
• Rest of Latin America
The Middle East & Africa Regulatory T-cells (Tregs) therapies Market, by Country, 2025-2030 (Value US$ Mn)
• GCC Countries
• South Africa
• Rest of the Middle East & Africa
Empower Your Decision-Making with 180 Pages Full Report @ https://www.insightaceanalytic.com/buy-report/1200
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory T-cells (Tregs) Therapies Market Key Players Analysis - Abata Therapeutics (US), Cellenkos Inc (US), Coya Therapeutics (US), Roche (Switzerland), Caladrius Biosciences (US). here
News-ID: 3788407 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd
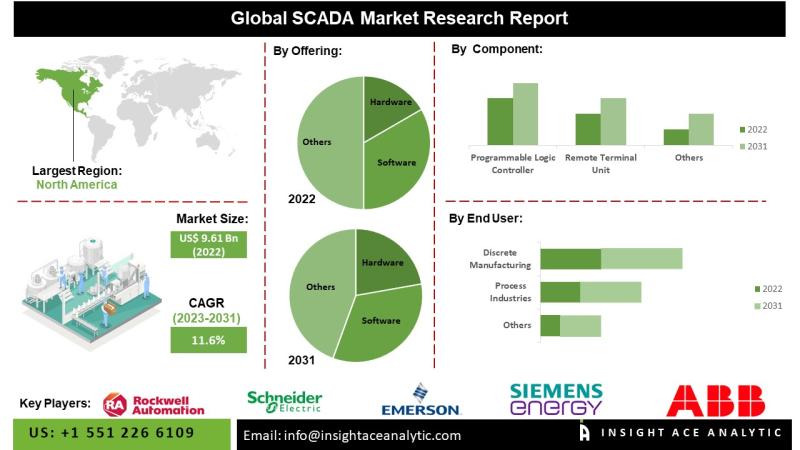
SCADA Market Insights Highlighting Technological Advancements in Wireless Sensor …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global SCADA Market Size, Share & Trends Analysis Report By Offering (Hardware, Software, Services), Component (Programmable Logic Controller, Remote Terminal Unit, Human-Machine Interface), End User (Process Industries, Discrete Manufacturing, Utilities), Region, Market Outlook And Industry Analysis 2034"
The global SCADA market is estimated to reach over USD 25.0 billion by the year 2034, exhibiting a CAGR of…
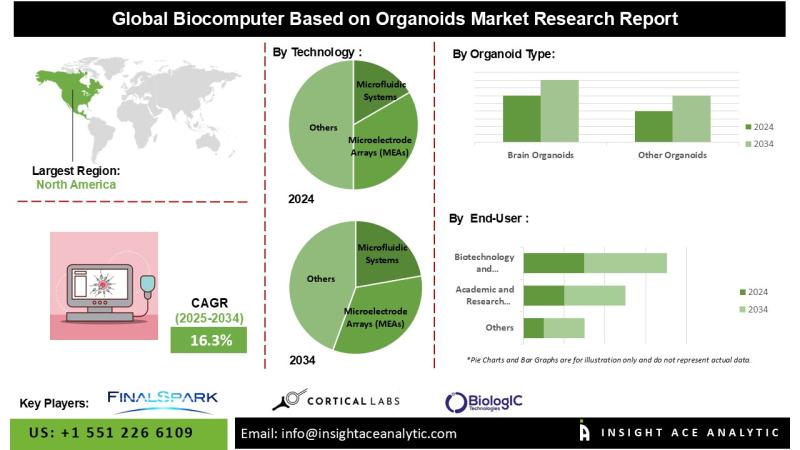
Biocomputer Based on Organoids Market Poised for 16.3% CAGR Driven by Brain Orga …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Biocomputer Based on Organoids Market Size, Share & Trends Analysis Report By Organoid Type (Brain Organoids, Other Organoids), Application (Biological Computing, Neuroscience Research, Drug Discovery and Testing, Personalized Medicine, Regenerative Medicine), Technology (Microfluidic Systems Microelectrode Arrays (MEAs), Brain-Machine Interfaces, CRISPR and Gene Editing), End-User (Academic and Research Institutes, Biotechnology and Pharmaceutical Companies, Technology Companies, Contract…
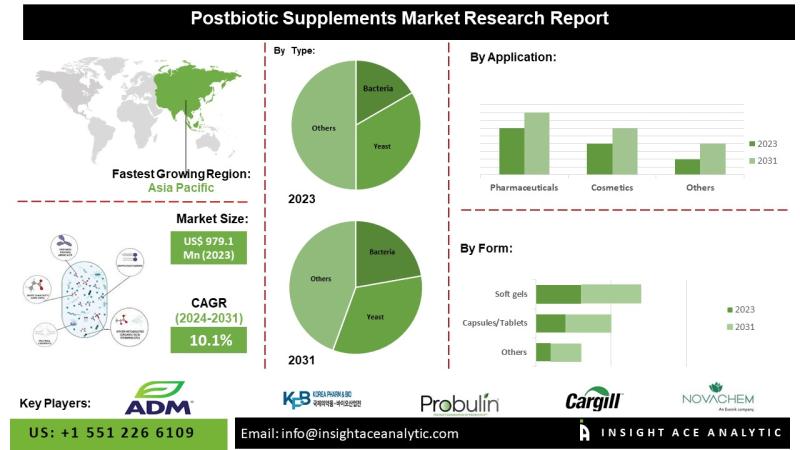
Postbiotic Supplements Market Drivers Include Functional Nutrition and Bioactive …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Postbiotic Supplements Market - (By Type (Bacteria, Yeast), By Form (Soft gels, Capsules/Tablets, Powder/ Granules, Others), By Application (Personal Care and Cosmetics, Food and Beverages, Animal Feed, Pharmaceuticals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Postbiotic Supplements Market is valued at USD 12.8…
Fused Deposition Modeling 3D Printing Market Trends Highlight Increasing Use in …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Fused Deposition Modeling 3D printing Market - (By Type (Stereolithography, Polyjet Printing, MultiJet Printing, Colorjet Printing, Digital Light Processing, Selective Laser Sintering), By Application (Consumer, Automotive, Aerospace & Defence, Healthcare, Fashion & Aesthetics)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Fused Deposition Modeling 3D…
More Releases for Treg
Treg Cell-Based Therapies Clinical Trial Landscape Expands With 55+ Emerging The …
The "Treg Cell-Based Therapies Pipeline Insight" report delivers a detailed assessment of therapeutic programs spanning the entire development continuum, from early discovery and preclinical research to advanced clinical-stage candidates. It features comprehensive drug profiles highlighting mechanisms of action, clinical trial progress, regulatory milestones, and partnerships, funding activities, and enabling technology platforms influencing product development.
DelveInsight's most recent evaluation underscores the accelerated global momentum in the Treg Cell-Based Therapies domain, with over…
Treg Therapy Market is Set to Experience a Revolutionary Growth
Regulatory T cells (Tregs) are a specialized subset of T cells that play a crucial role in maintaining immune homeostasis by suppressing excessive immune responses and preventing autoimmune diseases. Treg therapy, which involves the modulation or expansion of Tregs to restore immune balance, is emerging as a promising treatment for various autoimmune diseases, transplant rejection, and inflammatory disorders. Given their ability to control immune responses, Treg-based therapies hold great potential…
Tr1 Treg Treatment to Be Assessed in a Crohn's Disease Trial
The global Treg Therapy Market is rapidly gaining momentum as regulatory T-cell (Treg) therapies emerge as a transformative approach in immunology. Tregs are a specialized subset of T-cells that maintain immune tolerance and homeostasis, preventing the body from attacking its own tissues. Harnessing these cells offers a promising strategy to treat autoimmune diseases, organ transplant rejection, and chronic inflammatory conditions.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/72661
With advancements…
Treg Cell-Based Therapies Pipeline 2025: Latest FDA Approvals, Clinical Trials, …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Treg Cell-Based Therapies pipeline constitutes key 51+ companies continuously working towards developing 55+ Treg Cell-Based Therapies treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Treg Cell-Based Therapies Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The…
Treg Cell-Based Therapies Pipeline 2025: FDA Updates, Therapy Innovations, and C …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Treg Cell-Based Therapies pipeline constitutes key 51+ companies continuously working towards developing 55+ Treg Cell-Based Therapies treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Treg Cell-Based Therapies Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also…
Treg Cell-Based Therapies Clinical Trials Analysis 2025: EMA, PDMA, FDA Approval …
(Albany, USA) DelveInsight's "Treg Cell-Based Therapies Pipeline Insight, 2025" comprehensively analyzes the current clinical landscape and growth prospects in the Treg cell-based therapies market. The report covers disease insights, treatment guidelines, and a detailed pipeline assessment from preclinical to marketed stages. It includes drug mechanisms, clinical studies, regulatory progress, and key developments such as collaborations, mergers, funding, and designations.
For emerging Treg cell-based therapies drugs, the Treg cell-based therapies pipeline analysis…