Press release
Hemophilia A Clinical Trials 2024: EMA, PDMA, FDA Approvals, Medication, Therapies and Companies by DelveInsight | Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, etc
Hemophilia A companies are Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, Novo Nordisk, Hoffmann-La Roche, Chugai Pharmaceutical, Shire, Pfizer, BioMarin Pharmaceutical, Sinocelltech Ltd, Bayer, Ultragenix pharmaceutical, ApcinteX Ltd, Chia Tai Tianqing Pharmaceutical Group Co., Ltd, Expression Therapeutics, LLC, CSL Behring, ASC Therapeutics, Poseida Therapeutics, Staidson Beijing BioPharmaceuticals, Chia Tai Tianqing Pharmaceutical Group, and others.(Albany, USA) DelveInsight's 'Hemophilia A Pipeline Insight 2024' report provides comprehensive global coverage of available, marketed, and pipeline Hemophilia A therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the Hemophilia A pipeline domain.
For Hemophilia A emerging drugs, the Hemophilia A pipeline analysis report provides a 360° view of the therapeutics landscape by development point, product type, route of administration, molecule type, and MOA. The pipeline research covers business opportunities, challenges, future partnerships, strong competitors, and growth strategies.
To know more in detail about Hemophilia A pipeline report, click here: https://www.delveinsight.com/report-store/hemophilia-a-pipeline-insight-2020?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Key Takeaways from the Hemophilia A Pipeline Report
• Over 40+ Hemophilia A pipeline therapies are in various stages of development, and their anticipated acceptance in the Hemophilia A market would significantly increase market revenue.
• Leading Hemophilia A companies developing novel drug candidates to improve the Hemophilia A treatment landscape include Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, Novo Nordisk, Hoffmann-La Roche, Chugai Pharmaceutical, Shire, Pfizer, BioMarin Pharmaceutical, Sinocelltech Ltd, Bayer, Ultragenix pharmaceutical, ApcinteX Ltd, Chia Tai Tianqing Pharmaceutical Group Co., Ltd, Expression Therapeutics, LLC, CSL Behring, ASC Therapeutics, Poseida Therapeutics, Staidson Beijing BioPharmaceuticals, Chia Tai Tianqing Pharmaceutical Group, GeneVentiv Therapeutics, Jiangsu Gensciences, 2seventy bio, Generation Bio, Apitope Technology, and others.
• Promising Hemophilia A pipeline therapies in various stages of development include SPK-8011, SPK-8016, BIVV001, AGN-193408, Valoctocogene roxaparvovec, P-FVIII-101, STSP 0601, TQG203, SIG-001, Mim8, PF-07055480, Efanesoctocog alfa, WP 1301, Advate, Recombinate, antihemophilic factor, Eloctate, Hemlibra, Cyklokapron, NovoSeven RT, Xyntha, Humate-P, Hemofil-M, Adynovate, Afstyla, Feiba VH, Kogenate FS, Alphanate, Helixate FS, Autoplex T, Jivi, Nuwiq, Research program: gene therapies, GENV-HEM, Research program: gene editing therapeutics, NX-01, and others.
• In June of 2023, BioMarin disclosed that the FDA had granted approval to Roctavian for use as a single-dose therapy in individuals with severe hemophilia A.
• In February 2023, the FDA in the United States gave the green light to ALTUVIIIO (efanesoctocog alfa), a replacement therapy for factor VIII, for both adults and children diagnosed with hemophilia A. ALTUVIIIO is prescribed for regular preventive measures, as well as for addressing bleeding incidents when needed, and for managing the perioperative period (surgery) in adults and children suffering from hemophilia A.
Request a sample and discover the recent breakthroughs happening Hemophilia A pipeline landscape: https://www.delveinsight.com/sample-request/hemophilia-a-pipeline-insight-2020?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Hemophilia A Overview
Hemophilia is an inherited rare bleeding disorder in which blood does not clot properly because the affected person does not produce enough blood-clotting proteins (clotting factors). Patients who are injured cannot stop bleeding unless these factors are present. The severity of hemophilia symptoms is determined by the level of clotting factors. If the clotting factor level is mildly reduced, the patient may only bleed after surgery or trauma. If the deficiency is severe, one can easily bleed for no apparent reason. Small cuts are not dangerous, but internal bleeding is extremely dangerous for Hemophilia patients. Internal bleeding in the elbows, knees, ankles and other joints is the main concern with this genetic disorder. Internal bleeding can cause organ and tissue damage as well as be potentially fatal.
Hemophilia is classified into several types, including Hemophilia A, Hemophilia B, Hemophilia C, and Von Willebrand disease. Hemophilia is classified based on the presence of clotting factors, such as factor VIII in Hemophilia A and factor IX in Hemophilia B. Screening tests for hemophilia include the Complete Blood Count (CBC), Activated Partial Thromboplastin Time (APTT) Test, Prothrombin Time (PT) Test, Fibrinogen Test, and clotting factor tests.
Hemophilia A Pipeline Therapies and Key Companies
• Efanesoctocog alfa: Sanofi
• FRSW 107: Jiangsu Gensciences
• Mim8: Novo Nordisk
• SPK-8016: Spark Therapeutics
• OCTA101: Octapharma
• FRSW 117: Jiangsu Gensciences
• TQG203: Chia Tai Tianqing/Pharmaceutical Group
• STSP 0601: Staidson Beijing/BioPharmaceuticals
• P-FVIII-101: Poseida Therapeutics
Learn more about the Hemophilia A emerging pipeline therapies @ Hemophilia A Clinical Trials advancements: Hemophilia A Pipeline Insight - https://www.delveinsight.com/report-store/hemophilia-a-pipeline-insight-2020?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Hemophilia A Pipeline Therapeutics Assessment
By Product Type
• Monotherapy
• Combination Therapy
By Stage
• Discovery
• Pre-Clinical
• Phase I
• Phase II
• Phase III
• Pre-registration
By Route of Administration
• Oral
• Intravenous
• Subcutaneous
By Molecule Type
• Small molecules
• Gene Therapies
• Bispecific antibodies
• Recombinant proteins
• Fusion Proteins
• Coagulants
• Blood coagulation factor replacements
Request a sample and discover the recent breakthroughs happening Hemophilia A pipeline landscape: https://www.delveinsight.com/sample-request/hemophilia-a-pipeline-insight-2020?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Scope of the Hemophilia A Pipeline Report
• Coverage: Global
• Key Hemophilia A Companies: Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, Novo Nordisk, Hoffmann-La Roche, Chugai Pharmaceutical, Shire, Pfizer, BioMarin Pharmaceutical, Sinocelltech Ltd, Bayer, Ultragenix pharmaceutical, ApcinteX Ltd, Chia Tai Tianqing Pharmaceutical Group Co., Ltd, Expression Therapeutics, LLC, CSL Behring, ASC Therapeutics, Poseida Therapeutics, Staidson Beijing BioPharmaceuticals, Chia Tai Tianqing Pharmaceutical Group, GeneVentiv Therapeutics, Jiangsu Gensciences, 2seventy bio, Generation Bio, Apitope Technology, and others.
• Key Hemophilia A Pipeline Therapies: SPK-8011, SPK-8016, BIVV001, AGN-193408, Valoctocogene roxaparvovec, P-FVIII-101, STSP 0601, TQG203, SIG-001, Mim8, PF-07055480, Efanesoctocog alfa, WP 1301, Research program: gene therapies, GENV-HEM, Research program: gene editing therapeutics, NX-01, Advate, Recombinate, antihemophilic factor, Eloctate, Hemlibra, Cyklokapron, NovoSeven RT, Xyntha, Humate-P, Hemofil-M, Adynovate, Afstyla, Feiba VH, Kogenate FS, Alphanate, Helixate FS, Autoplex T, Jivi, Nuwiq and others.
Dive deep into rich insights for Hemophilia A emerging therapies and assessment; visit @ Hemophilia A Therapeutic Assessment: https://www.delveinsight.com/sample-request/hemophilia-a-pipeline-insight-2020?utm_source=openpr&utm_medium=pressrelease&utm_campaign=apr
Table of Contents
1. Introduction
2. Executive Summary
3. Hemophilia A Overview
4. Hemophilia A Pipeline Therapeutics
5. Late Stage Products (Phase III)
6. Mid Stage Products (Phase II)
7. Early Stage Products (Phase I/II)
8. Preclinical Stage Products
9. Discovery Stage Products
10. Hemophilia A Therapeutic Assessment
11. Inactive Hemophilia A Products
12. Collaborations Assessment- Licensing / Partnering / Funding
13. Hemophilia A Unmet Needs
14. Hemophilia A Market Drivers and Barriers
15. Appendix
16. About DelveInsight
Trending Reports
• Central Serous Chorioretinopathy Market: https://www.delveinsight.com/report-store/central-serous-chorioretinopathy-market
• Chronic Hepatitis B Virus Market: https://www.delveinsight.com/report-store/chronic-hepatitis-b-virus-market
• Chronic Hepatitis Delta Virus Market: https://www.delveinsight.com/report-store/chronic-hepatitis-delta-virus-infection-market
• Chronic Idiopathic Urticaria Market: https://www.delveinsight.com/report-store/chronic-spontaneous-urticaria-market
• Chronic Refractory Gout Market: https://www.delveinsight.com/report-store/chronic-refractory-gout-market
• Condyloma Market: https://www.delveinsight.com/report-store/genital-warts-condyloma-acuminatum-market
• Contraceptive Devices Market: https://www.delveinsight.com/report-store/contraceptive-devices-market
• Contusion Market: https://www.delveinsight.com/report-store/contusion-market
• Cough Assisted Device Market: https://www.delveinsight.com/report-store/cough-assist-devices-market
• Dermal Mycosis Market: https://www.delveinsight.com/report-store/dermal-mycosis-market
• Encephalitis Market: https://www.delveinsight.com/report-store/encephalitis-market
• Endometriosis Pain Market: https://www.delveinsight.com/report-store/endometriosis-pain-market
• Generalized Pustular Psoriasis Market: https://www.delveinsight.com/report-store/generalized-pustular-psoriasis-market
• Giant Papillary Conjunctivitis Market: https://www.delveinsight.com/report-store/giant-papillary-conjunctivitis-market
• Graves' Disease Market: https://www.delveinsight.com/report-store/graves-disease-market
• Hepatic Encephalopathy Epidemiology Forecast: https://www.delveinsight.com/report-store/hepatic-encephalopathy-epidemiology-forecast
• Hepatic Encephalopathy Market: https://www.delveinsight.com/report-store/hepatic-encephalopathy-market
• Hereditary Angioedema Market: https://www.delveinsight.com/report-store/hereditary-angioedema-market
• Hyperinsulinemic Hypoglycemia Market: https://www.delveinsight.com/report-store/congenital-hyperinsulinism-market
• Lymphocytopenia Market: https://www.delveinsight.com/report-store/lymphocytopenia-market
• Malt Lymphoma Market: https://www.delveinsight.com/report-store/marginal-zone-lymphoma-market
• Nmibc Market: https://www.delveinsight.com/report-store/non-muscle-invasive-bladder-cancer-market
• Parkinsons Disease Related Dementia Market: https://www.delveinsight.com/report-store/parkinsons-disease-related-dementia-market
• Phosphoglucomutase Pgm 1 Deficiency Market: https://www.delveinsight.com/report-store/phosphoglucomutase-pgm-1-deficiency-market
• Radioligand Therapies Market: https://www.delveinsight.com/report-store/radioligand-therapies-market-forecast
Contact Us:
Ankit Nigam
Manager Marketing
info@delveinsight.com
+14699457679
https://www.delveinsight.com/oncology
Case study: https://www.delveinsight.com/case-study/competitive-landscape-assessment
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Hemophilia A Clinical Trials 2024: EMA, PDMA, FDA Approvals, Medication, Therapies and Companies by DelveInsight | Spark Therapeutics, Sigilon Therapeutics, ASC Therapeutics, Pfizer, Sanofi Genzyme, etc here
News-ID: 3756760 • Views: …
More Releases from DelveInsight Business Research
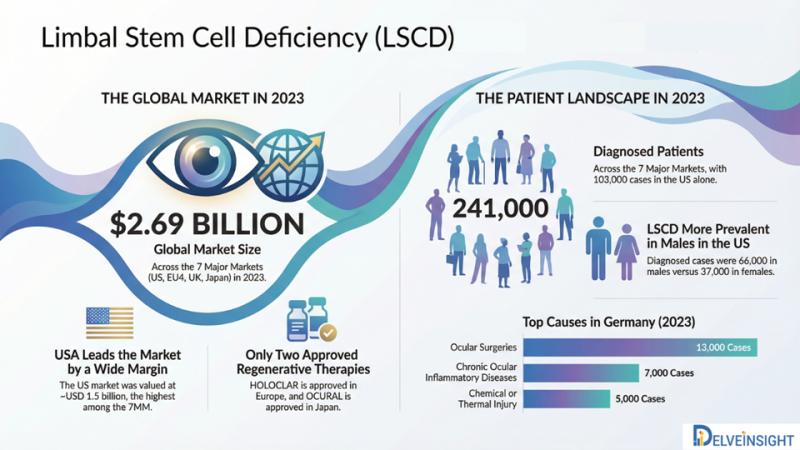
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
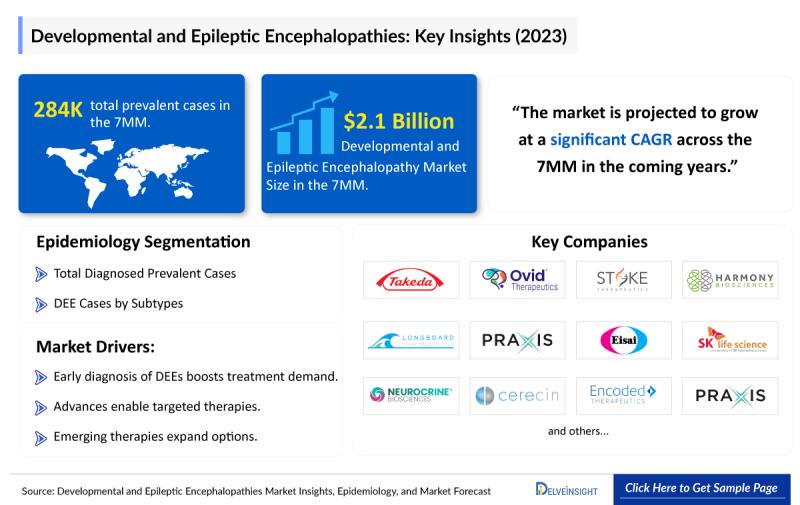
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerat …
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.
The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs…
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
More Releases for Hemophilia
Rising Occurrence Of Genetic Abnormalities Fuels Hemophilia Market Growth: Stren …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts
What Is the Expected CAGR for the Hemophilia Market Through 2025?
In recent times, there has been a significant expansion in the hemophilia market. The market, which stood at $13.83 billion in 2024, is projected to inflate to $15.13 billion in 2025, exhibiting a compound annual growth rate (CAGR)…
Global Hemophilia Market Size & Trends
According to a new market research report published by Global Market Estimates, the global hemophilia market is projected to grow at a CAGR of 6.8% from 2023 to 2028.
During the forecast period, the global hemophilia market is anticipated to experience growth due to advancements in medical research, an increase in hemophilia incidence, and rising awareness and diagnosis.
Browse 147 Market Data Tables and 115 Figures spread through 163 Pages and…
Acquired Hemophilia Treatment Market - Embracing Hope, Defying Hemophilia: Trans …
Newark, New Castle, USA: The "Acquired Hemophilia Treatment Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Acquired Hemophilia Treatment Market: https://www.growthplusreports.com/report/acquired-hemophilia-treatment-market/7748
This latest report researches the industry structure,…
Hemophilia Market
Hemophilia is an inherited genetic syndrome which impairs body-2019s capability to control blood clotting or coagulation. The episodes of bleeding mainly depend on the severity of hemophilia issue. There are two major kinds of hemophilia namely Hemophilia A & Hemophilia B. Hemophilia A is considered to be five times more prevailing as compared to hemophilia B. Occurrence of hemophilia A is found in one out of five or six thousand…
Global Hemophilia Therapeutics Market By Drug (Advate, NovoSeven, Kogenate/Koval …
ReportsWorldwide has announced the addition of a new report title Global Hemophilia Therapeutics Market By Drug (Advate, NovoSeven, Kogenate/Kovaltry, Feiba), By Type of Hemophilia (Hemophilia A, Hemophilia B), By Treatment (On-Demand, Prophylaxis), By Therapy (Replacement , Immune Tolerance Induction ) Outlook 2022 to its growing collection of premium market research reports.
Hemophilia is an inherited bleeding disorder that slows the blood clotting process. The people suffering from this condition experience prolonged…
In-depth analysis of the Global Hemophilia Market: hemophilia A, hemophilia B, h …
Latest industry research report on: Global Hemophilia Market | Industry Size, Share, Research, Reviews, Analysis, Strategies, Demand, Growth, Segmentation, Parameters, Forecasts
Request For Sample Report @ http://www.marketresearchreports.biz/sample/sample/1064504
The hemophilia market has entered into an era of unprecedented growth rate with a variety of treatment options. Development of new therapeutics along with expansion in the current treatment options has presented new opportunities to the market. Hemophilia being an inherited genetic bleeding disorder causes…