Press release
Regulatory Affairs Outsourcing Market Projected to Grow at USD 15.5 billion with Top Players | Genpact (U.S.), CRITERIUM, INC. (U.S.), PRA Health Sciences (U.S.), Promedica International (U.S.) and Forecast to 2031
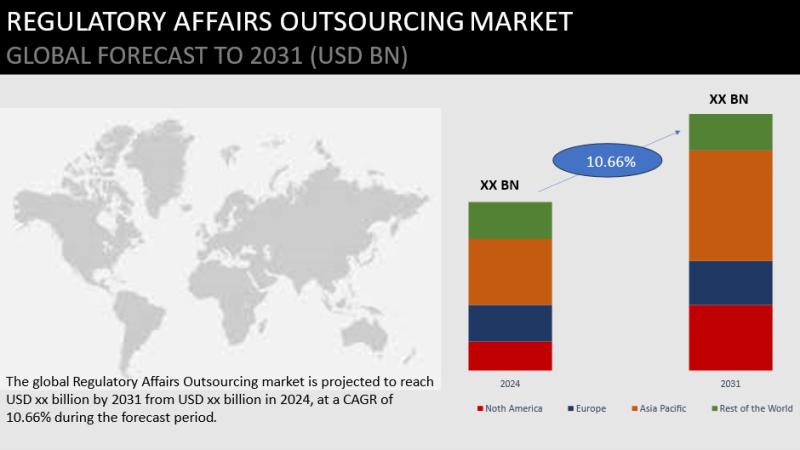
Global Regulatory Affairs Outsourcing Market in terms of revenue expected to reach USD 15.5 billion and is poised to grow at a CAGR of 10.66% to Forecast 2031, The regulatory affairs outsourcing market is primarily driven by a notable rise in fixed in-house costs related to activities such as training, technology, specialized knowledge, and facilities. Additionally, the increasing number of clinical trials contributes to this market's growth. Furthermore, there is an opportunity for the market due to improvements in pre-marketing and post-marketing processes, leading to reduced regulatory service costs.Get Free Sample Report@ https://www.theresearchinsights.com/request_sample.php?id=559034
Increasing out-of-pocket expenses in both developed and developing nations, along with uneven economic growth and government efforts to reduce drug costs, are factors contributing to economic and competitive pressures. This is anticipated to drive demand for regulatory affairs outsourcing among life science companies. Early-stage product-specific clinical guidance and regulatory compliance are crucial for product approval. Failure to address regulatory compliance early in development often results in approval delays due to inadequately designed studies, manufacturing oversights, omitted studies, and other regulatory requirement failures.
Regulatory Affairs Outsourcing Market Companies:
Accell Clinical Research, LLC
Genpact
CRITERIUM, INC
PrimeVigilance
Freyr
PHARMALEX GMBH
NDA Group AB
ProPharma Group MIS Limited
Regulatory Pharma Net srl
ICON plc
Labcorp Drug Development
Parexel International Corporation
Pharmexon
APCER Life Sciences, Inc.
Real Regulatory Ltd
VCLS
REGENOLD GMBH
Market Segmentation:
By Service
Legal Representation
Product Registration & Clinical Trial Application
Regulatory Writing & Publication
Regulatory Consulting
Others
By Category
Medical Devices
Biologics
Drugs
By End User
Pharmaceutical Company
Medical Device Company
Biotechnology Company
By Indication
Oncology
Immunology
Cardiology
Neurology
Others
By Stage
Clinical
Preclinical
Post Market Authorization
Get Exclusive Discount@ https://www.theresearchinsights.com/ask_for_discount.php?id=559034
Regulatory Affairs Outsourcing Market Dynamics
Drivers:
Increasing Regulatory Complexity
Growing complexity in pharmaceuticals, biotechnology, and medical devices drives companies to seek specialized regulatory expertise from outsourcing partners for efficient compliance.
Rapid Technological Advances
Continuous innovation in industries like pharmaceuticals and medical devices necessitates outsourcing partners with specialized knowledge to navigate regulatory hurdles and accelerate product launch.
Focus on Core Competencies
Companies prioritize their core competencies, such as R&D and marketing, by outsourcing regulatory affairs tasks to experts, enabling more efficient resource allocation.
Flexibility and Scalability
The outsourcing model offers flexibility and scalability, allowing companies to adjust regulatory operations based on project needs, ensuring efficient resource utilization.
Key Developments:
In Jan 2023, AmerisourceBergen Corporation expanded its service portfolio by acquiring PharmaLex Holding GmbH, a leading provider of regulatory affairs services in the life sciences industry.
In Apr 2022, VCLS partnered with EC Innovations, a global translation services company with expertise in translating regulated life sciences and medical content. This collaboration aimed to enhance VCLS's offerings and operational capabilities.
Table of Contents
Executive Summary
Market Introduction
Research Methodology
Market Dynamics
Regulatory Affairs Outsourcing Market ANALYSIS by SEGMENT (COUNTRY LEVEL ANALYSIS)
NORTH AMERICA Regulatory Affairs Outsourcing Market analysis
EUROPE Regulatory Affairs Outsourcing Market analysis
ASIA PACIFIC Regulatory Affairs Outsourcing Market analysis
Rest of the World Regulatory Affairs Outsourcing Market analysis
Company Profiles
Buy Complete Report@ https://www.theresearchinsights.com/checkout?id=559034
Contact Us:
Robin
Sales Manager
+ 91-996-067-0000
+312-313-8080
sales@theresearchinsights.com
About US:
The Research Insights - A world leader in analysis, research and consulting that can help you renew your business and change your approach. With us you will learn to make decisions with fearlessness. We make sense of inconveniences, opportunities, circumstances, estimates and information using our experienced skills and verified methodologies. Our research reports will provide you with an exceptional experience of innovative solutions and results. We have effectively led companies around the world with our market research reports and are in an excellent position to lead digital transformations. Therefore, we create greater value for clients by presenting advanced opportunities in the global market.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market Projected to Grow at USD 15.5 billion with Top Players | Genpact (U.S.), CRITERIUM, INC. (U.S.), PRA Health Sciences (U.S.), Promedica International (U.S.) and Forecast to 2031 here
News-ID: 3467029 • Views: …
More Releases from The Research Insights

Latest Research Report on Travel Services Market by Forecast to 2032
The Global Travel Services Market has experienced substantial growth, driven by increasing globalization, rising disposable incomes, technological advancements, and growing consumer preference for experiential travel. The market encompasses a wide range of services, including travel planning, transportation, accommodation, and tourism activities. This article provides a detailed analysis of the market size, share, trends, and growth prospects, highlighting the factors shaping the industry's future through 2032.
𝐂𝐥𝐢𝐜𝐤 𝐭𝐡𝐞 𝐥𝐢𝐧𝐤 𝐭𝐨 𝐠𝐞𝐭 𝐚…
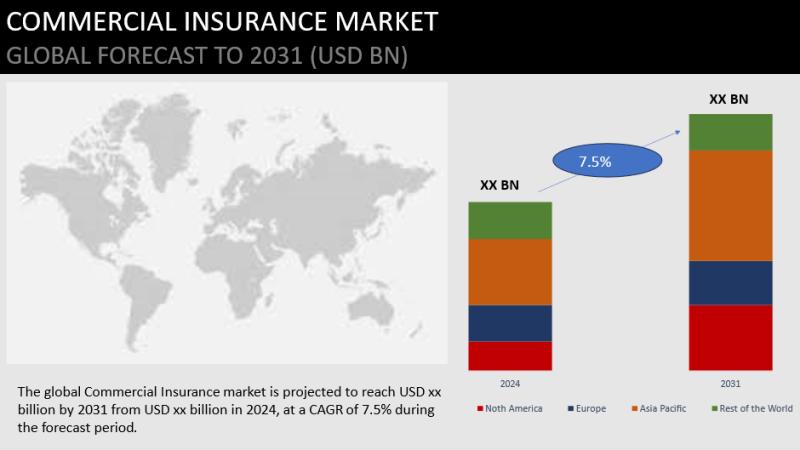
Commercial Insurance Market is Expected to Grow at a CAGR 2024 - 2031
The Commercial Insurance Insights of 2024 is a thorough analysis that offers a thorough look at the market's size, revenues, market shares, various categories, drivers, and other pertinent factors in addition to trends and possible new developments. Aside from the 2031 forecast period, the research study discusses market restraints and regional industrial presence that may impact market growth patterns. In PDF version, the Commercial Insurance Market study comprises an extensive…
Portable Power Station Market Forecast 2024-2031 - 46.6% CAGR Growth by 2031
The Portable Power Station Insights of 2024 is a thorough analysis that offers a thorough look at the market's size, revenues, market shares, various categories, drivers, and other pertinent factors in addition to trends and possible new developments. Aside from the 2031 forecast period, the research study discusses market restraints and regional industrial presence that may impact market growth patterns. In PDF version, the Portable Power Station Market study comprises…
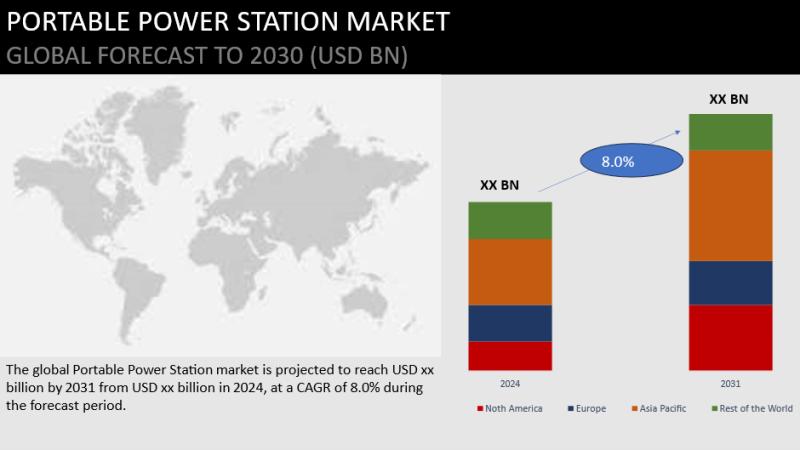
Portable Power Station Market To Grow at a Stayed CAGR from 2024 to 2031
The Portable Power Station Insights of 2024 is a thorough analysis that offers a thorough look at the market's size, revenues, market shares, various categories, drivers, and other pertinent factors in addition to trends and possible new developments. Aside from the 2031 forecast period, the research study discusses market restraints and regional industrial presence that may impact market growth patterns. In PDF version, the Portable Power Station Market study comprises…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…