Press release
Regulatory Affairs Outsourcing Market to Reach USD 17.3 Billion by 2028
In a recent study conducted by Transparency Market Research, the global regulatory affairs outsourcing market has been projected to grow significantly, reaching a value of US$ 17.3 billion by the end of 2028. The market, valued at US$ 4.5 billion in 2020, is estimated to expand at a remarkable Compound Annual Growth Rate (CAGR) of 19% from 2021 to 2028.The surge in research and development activities within the life sciences industry worldwide is identified as a primary driver propelling the global market during the forecast period. Factors such as the increasing number of clinical trials, advancements in biosimilars, personalized medicine, and orphan pharmaceuticals contribute to this growth trajectory.
Get Exclusive PDF Sample Copy of Regulatory Affairs Outsourcing Market Report
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528
Key findings from the report include:
Drivers of Growth: The rise in investment in Research & Development activities aimed at developing biosimilar, orphan, and generic drugs, coupled with a heightened focus on product development to expand supply, are key drivers boosting the global regulatory affairs outsourcing market. Additionally, the increase in the number of FDA-approved manufacturing plants signifies growth opportunities within the industry.
Market Opportunities: The report identifies significant opportunities for both global and mid-sized market players, including the delivery of low-cost regulatory services and intensified marketing activities. Despite the challenges posed by the COVID-19 pandemic, which has placed pressure on regulatory and outsourcing teams, there has been a positive effect on the bio/pharmaceutical outsourcing industry, with growing demand for R&D activities leading to increased demand for regulatory affairs assistance.
Risk Factors: Risks associated with outsourcing and data sharing are identified as potential impediments to market growth. Concerns about sensitive data access by outsourcing partners pose threats to data security for companies operating within the industry.
The report profiles major players in the global regulatory affairs outsourcing market, including Accell Clinical Research, LLC, Charles River Laboratories International, Inc., Clinilabs, Inc., Covance, Inc., and others. These profiles encompass company overviews, financial summaries, product portfolios, business strategies, and recent developments.
Key developments in the market include:
Freyer's development of new regulatory affairs outsourcing software, offering end-to-end regulatory document management solutions.
Parexel International Corporation's expansion of regulatory services through medical writing offerings.
Charles River Laboratories International, Inc.'s acquisition of Citoxlab, aimed at broadening the company's product offering and enhancing market position.
Buy this premium research report
https://www.transparencymarketresearch.com/checkout.php?rep_id=3528
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Nikhil Sawlani
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market to Reach USD 17.3 Billion by 2028 here
News-ID: 3394036 • Views: …
More Releases from Transparency Market Research
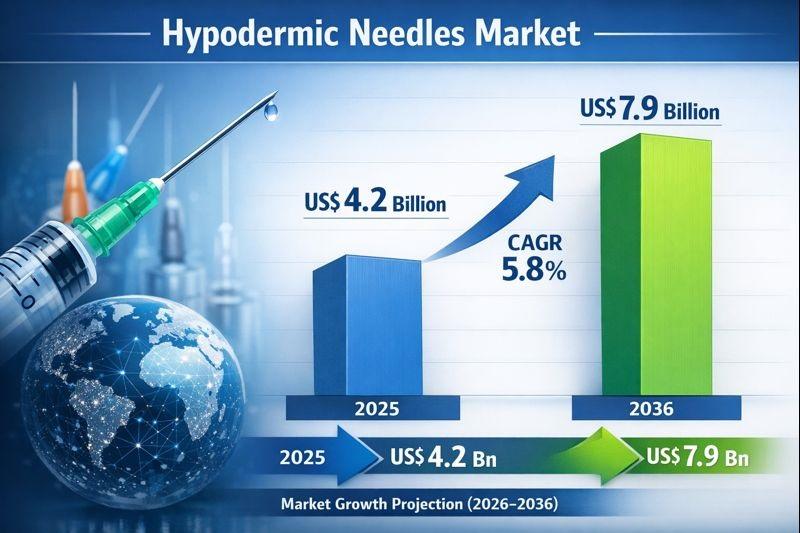
Hypodermic Needles Market to Reach US$ 7.9 Billion by 2036 on Rising Injectable …
The global hypodermic needles market was valued at approximately US$ 4.2 billion in 2025 and is projected to reach around US$ 7.9 billion by 2036, expanding at a CAGR of nearly 5.8% from 2026 to 2036, driven by the rising prevalence of diabetes, cancer, and chronic diseases, growing demand for injectable drugs and biologics, and the expansion of global vaccination and immunization programs; increasing adoption of safety-engineered and disposable needles,…
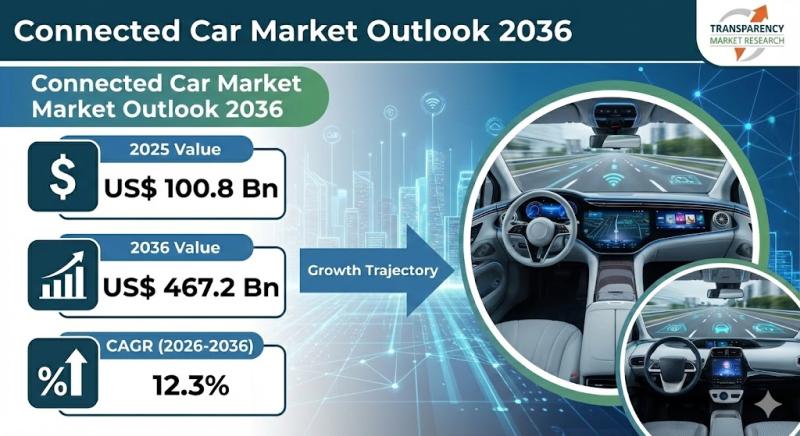
Connected Car Market to Reach US$ 467.2 Billion by 2036, Driven by Rising Adopti …
The global connected car market is entering a high-growth phase as vehicles increasingly evolve into software-defined, data-driven mobility platforms. Valued at US$ 100.8 billion in 2025, the market is projected to reach an impressive US$ 467.2 billion by 2036, expanding at a robust CAGR of 12.3% from 2026 to 2036. This growth is fueled by rapid advancements in automotive connectivity, rising consumer demand for intelligent features, and strong integration of…
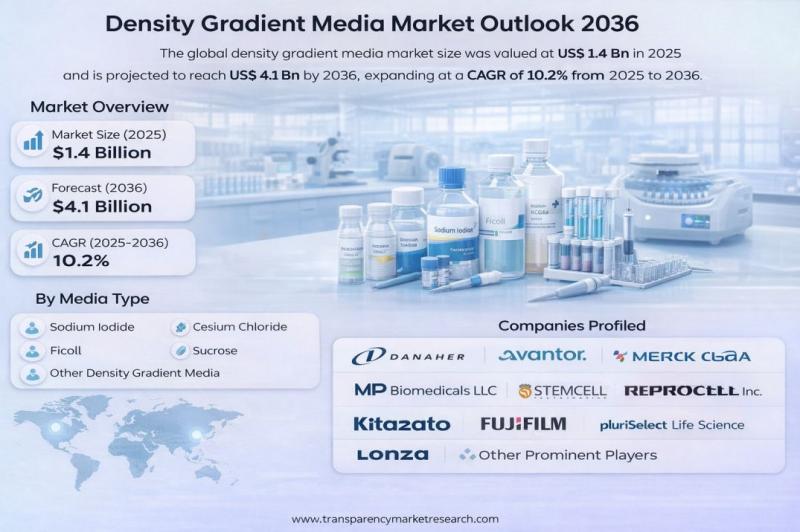
Density Gradient Media Market to Reach US$ 4.1 Billion by 2036, Driven by Rapid …
The global density gradient media market was valued at US$ 1.4 Billion in 2025 and is projected to reach US$ 4.1 Billion by 2036, expanding at a robust CAGR of 10.2% from 2025 to 2036. The market's rapid growth is primarily driven by increasing demand for rapid infectious disease screening, expanding cell therapy and immunology research, and continuous technological advancements improving sensitivity and multiplexing in laboratory workflows.
Access key findings and…
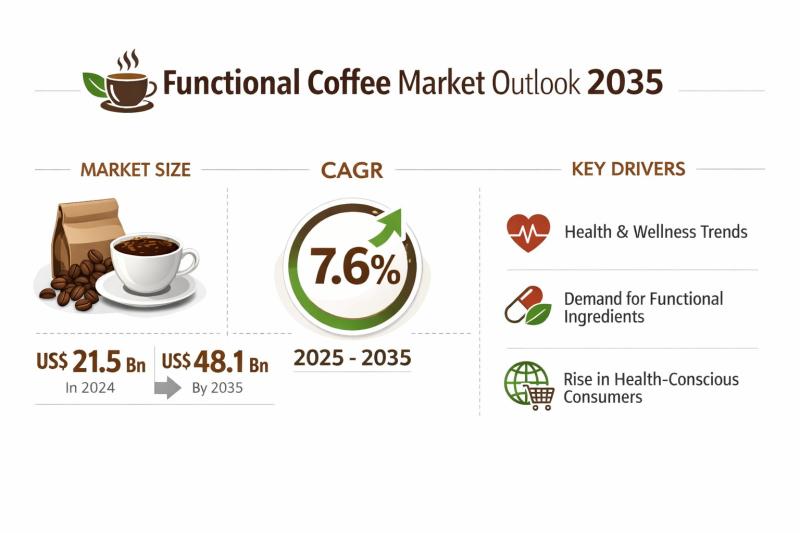
Functional Coffee Market Expanding at 7.6% CAGR Through 2035 - By Product Type / …
The global functional coffee market was valued at US$ 21.5 Bn in 2024 and is projected to reach US$ 48.1 Bn by 2035, expanding at a compound annual growth rate (CAGR) of 7.6% from 2025 to 2035. This steady growth trajectory reflects the strong convergence of coffee consumption habits with rising demand for functional and wellness-oriented beverages. Functional coffee has transitioned from a niche category to a mainstream product offering,…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…