Press release
Pharmacovigilance and Drug Safety Software Market research report provides an integrated information on the major drivers, restraints and opportunities influencing the industry growth during 2018-2023.
With collective industry experience of about 200 years of its analysts and experts, Allied Market Research (AMR) encompasses most infallible research methodology for its market intelligence and industry analysis. We do not only engrave the deepest levels of markets but also sneak through its slimmest details for the purpose of our market estimates and forecasts. Our approach helps in building greater market consensus view for size, shape and industry trends within each industry segment.Get sample Report: https://www.alliedmarketresearch.com/request-toc-and-sample/922?utm_source=openpr
Drug safety management tool or software, that aids in reviewing, classifying, creating and other pharmacovigilance data is termed as PV software (or Pharmacovigilance software). The software also helps in the creation of adverse event reports. Furthermore, the PV software is majorly used by BPOs, contract research organizations (CROs ) and major pharmacovigilance solution providers.
There are several drivers, restraints and opportunities shaping the future of the market. Important factors including growing incidences of ADRs have played a major role in driving the pharmacovigilance and drug safety software market. Furthermore, rising adoption rate of such software by many outsourcing companies too have kept the future of the market bright. On the other hand, government entities including FDA, EMEA and others, has increased the pressure on the biotechnology and pharmaceutical brands to manufacture safe drugs. The aforesaid factor has also added to the increasing market share of the industry. However, lack of awareness about the benefits associated with the pharmacovigilance and drug safety software market has hampered its market growth. Regions such as Asia Pacific and Latin America are likely to create greater opportunities for the market.
Purchase an enterprise user license and get 1 month complimentary access to our Knowledge Tree subscription. (*TC)
Get subscription: https://www.alliedmarketresearch.com/checkout/182712
The market is segmented based on functions, delivery model, end-customers and geography. Based on function the market segments discussed in the report consists of issue tracking solution, fully integrated solution, adverse event reporting solution etc. The mode of delivery assessed in the market research report includes on -demand and on premise delivery. The major end-customers examined during the study are pharma companies, CROs, BPOs and others. Geographies promising growth includes North America, Asia Pacific, Europe and LAMEA.
A closer look at the competitive landscape reveals that major IT brands are busy launching pharma co-vigilance and drug safety software and generating clients in the same month itself. Prominent market players are also seen acquiring new companies or local market players to maintain their competitive edge. Other business strategies favored by the companies include constant up-gradation, joint ventures and collaborations. Prominent market players discussed in the market research report are Oracle Corporation, Online Business Applications, Inc., Sparta Systems, Inc., United Bio-source Corporation and others.
For Purchase Inquiry: https://www.alliedmarketresearch.com/purchase-enquiry/922?utm_source=openpr
About Us:-
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Portland, Oregon. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions.” AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
Contact Us:-
David Correa
5933 NE Win Sivers Drive
#205, Portland, OR 97220
United States
USA/Canada (Toll Free):
+1-800-792-5285, +1-503-894-6022, +1-503-446-1141
UK: +44-845-528-1300
Hong Kong: +852-301-84916
India (Pune): +91-20-66346060
Fax: +1(855)550-5975
help@alliedmarketresearch.com
Web: https://www.alliedmarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance and Drug Safety Software Market research report provides an integrated information on the major drivers, restraints and opportunities influencing the industry growth during 2018-2023. here
News-ID: 1417548 • Views: …
More Releases from Allied Market Research
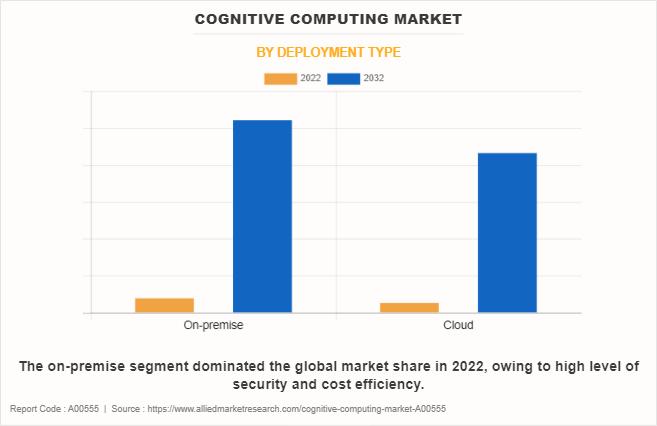
Cognitive Computing Market Set to Achieve a Valuation of $476.8 Billion by 2032, …
According to a new report published by Allied Market Research, titled, "Cognitive Computing Market, by Deployment Type (On-premise, Cloud), by Technology (Natural Language Processing (NLP), Machine Learning, Automated Reasoning, Others), by Enterprise Size (Large Enterprises, Small and Medium-sized Enterprises), by Industry Vertical (Healthcare, BFSI, Retail and Ecommerce, Government and Defense, IT and Telecom, Energy and Power, Others): Global Opportunity Analysis and Industry Forecast, 2023-2032"
The cognitive computing market was valued at…
Neuromorphic Chip Market Size is is projected to reach $7.1 billion by 2032 | Br …
Allied Market Research published an exclusive report, titled, "Neuromorphic Chip Market Size, Share, Competitive Landscape and Trend Analysis Report by End user : Global Opportunity Analysis and Industry Forecast, 2023-2032".
The Global Neuromorphic Chip Market was valued at $87.9 million in 2022, and is projected to reach $7.1 billion by 2032, growing at a CAGR of 55.8% from 2023 to 2032.
Download Research Report Sample & TOC : https://www.alliedmarketresearch.com/request-sample/2345
The neuromorphic chip…

Digital Payment Market Reach USD 457.8 Trillion by 2032, Key Factors behind Mark …
Allied Market Research published a new report, titled, " The Digital Payment Market Reach USD 457.8 Trillion by 2032, Key Factors behind Market's Exponential Growth." The report offers an extensive analysis of key growth strategies, drivers, opportunities, key segment, Porter's Five Forces analysis, and competitive landscape. This study is a helpful source of information for market players, investors, VPs, stakeholders, and new entrants to gain thorough understanding of the industry…

API Management Market Reach USD 41.5 Billion by 2031 at a CAGR of 34.5%, Key Fac …
Allied Market Research published a new report, titled, " The API Management Market Reach USD 41.5 Billion by 2031 at a CAGR of 34.5%, Key Factors behind Market's Exponential Growth." The report offers an extensive analysis of key growth strategies, drivers, opportunities, key segment, Porter's Five Forces analysis, and competitive landscape. This study is a helpful source of information for market players, investors, VPs, stakeholders, and new entrants to gain…
More Releases for Drug
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…
Microcapsules Drug Delivery Market Microcapsules Drug Delivery Sales Report 2022
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Indispensable Advent of Microcapsules
1.1 Trajectory of Microencapsulation
1.2 Why and wherefores for Microencapsulation
2. Characterization of Microcapsules
2.1 Composition of Microcapsules
2.2 Parameters Influencing Microcapsules
3. Engineering Technology of Microcapsules
3.1 Physical Manufacturing Technologies
3.2 Physicochemical and Chemical Technologies
4. Applicability of Microcapsules
4.1 Microcapsules in Pharmaceuticals
4.2 Microcapsules in Nutraceuticals
…
AstraZeneca’s NMOSD Drug receives orphan drug designation
April 18, 2017 Orphan Drug
European Medicine Agency has Announced orphan drug designation for inebilizumab (earlier known as MEDI-551) developed by AstraZeneca PLC (AZN) for the treatment of neuromyelitis optica spectrum disorder (NMOSD). The drug has already received orphan drug status from US Food and Drug Administration (FDA). NMOSD, also called Devic’s disease, is a rare, autoimmune disorder of the central nervous system (CNS) that affects the functioning…
Drug Discovery Strategies, PBPK Modeling and Drug-Drug Interaction Explored and …
This year’s 12th annual ADMET conference and exhibition promises to deliver appearances from experienced pharmaceutical professionals from ADME and PK/PD organisations such as AbbVie, GSK, Roche and Bayer when it returns to London this June.
The carefully selected speaker line up will be addressing early ADME application strategies and discussing the latest technologically advanced screening and testing models along with exploring ADMET technologies which will identify properties of a drug…
