Press release
Clinical Trial Management System Market 2018 Competitive Landscape Analysis with Trends, Application, Growth, Technology, Analysis and Forecast to 2025
High cost involved with launch of new drugs along with extended timelines has fostered the need to improve operational efficiency of clinical trials. Increasing reliance of industry sponsors on advanced IT-based clinical trial management solutions to improve managerial control in clinical trials will boost CTMS market growth. Outsourcing of clinical trials to contract research organizations offering quality of services will spur market size. However, prohibitive cost of clinical trial management systems will hinder industry growth over the forecast timeline.The Global Clinical Trial Management System Market is expected to reach USD 1508.7 Million by 2025 from USD 588 Million in 2017 growing at a CAGR of 12.50% during the forecast period of 2018 to 2025. The upcoming market report contains data for historic years 2017, the base year of calculation is 2017 and the forecast period is 2018 to 2025
Get Free Sample copy of report at http://databridgemarketresearch.com/request-a-sample/?dbmr=global-clinical-trial-management-system-market
Some of the major players operating in the global clinical trial management system market are Bioclinica, Oracle Corporation, eClinForce Inc., Medidata Solutions, DATATRAK International, Inc, Guger Technologies Inc, PARAXEL International Corporation, MedNet Solutions, Inc. ChemWare Inc., iWEb Technologies, Data MATRIX, Jade Global Solutions, Integrated Clinical Solutions, MAJARO InfoSystems, BioOptronics, Inc, Forte Research Systems and ICON plc, Merge healthcare incorporated, Bio-Optronics, DSG INC, eClinForce, ArisGlobal, ERT Clinical and among others.
Clinical trial management system (CTMS) involves management of clinical trial records in clinical research. CTMS starts from planning of research proposal to preparation, conduct of trial, report generation, CTMS maintenance and managing the clinical trial data. CTMS reduces the communication period for a multi-centered clinical trial. The main advantage of CTMS system is that it sponsors or client can access their trial progress from anywhere. CTMS provides the valuable information like Clinical trial program/ project management, trial and site planning site and subject management, study management, investigator management, study financials and investigator grants and payments management lastly clinical trial performance and reporting. In 2004, International Committee for Medical Journal Editors (ICMJE) made it mandatory to fulfill all publications required for study for the clinical trial registration. As per of outsourcing Pharma 2014, out of the 111 clinical studies listed under U.S. FDA, more than 70 were handled by CROs. Since 2006, Massey Cancer Center by utilizing the CTMS Platform securely accesses the clinical trial progress via internet. In August 2016, Bioclinica announced the complete acquired sition of Cinven. Through this agreement Bioclinica expand its services and products and strategies in the CTMS. In September 2017, Pamplona Capital Management, LLP completed acquisition of PAREXEL. This agreement allowed better growth and deep healthcare expertise and great achievement in healthcare sector.
Access More Details at http://databridgemarketresearch.com/reports/global-clinical-trial-management-system-market/
The global clinical trial management system market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions and others to increase their footprints in this market. The report includes market shares of clinical trial management system market for global, Europe, North America, Asia Pacific and South America.
Major Market Drivers and Restraints:
- Growth of healthcare IT sector.
- Increase in the research and development expenditure in the life sciences
- Growth in clinical research organization
- High prevalence of chronic diseases
- Increase adoption of CTMS solutions
- Advancement in technology and software for clinical trial management system
- Synchronization of hospital information system (HIS) with CTMS
Strict regulations for clinical trial protocols
Inquire before buying at http://databridgemarketresearch.com/inquire-before-buying/?dbmr=global-clinical-trial-management-system-market
Note: If you have any special requirements, please let us know and we will offer you the report as you want.
Contact Info:
Name: Data Bridge Market Research
Email: sopan.gedam@databridgemarketresearch.com
Phone: +1 888 387 2818
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Amanora Chambers,
Magarpatta Road, Hadapsar
Pune – 411028
Maharashtra, India
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management System Market 2018 Competitive Landscape Analysis with Trends, Application, Growth, Technology, Analysis and Forecast to 2025 here
News-ID: 1050119 • Views: …
More Releases from Data Bridge Market Research

Vitamin K Market IS growing at a CAGR of 6.8% during the forecast period of 2023 …
Vitamin K Market Analysis and Size
The essential factors contributing to the growth of the market in the forecast period of 2023 to 2020 include growing demand for vitamin supplements, changing dietary patterns, and rising health consciousness.
Data Bridge Market Research analyses that the global vitamin K market, which was USD 841.47 million in 2022, is expected to reach USD 1,410.20 million by 2030, growing at a CAGR of 6.8% during the…
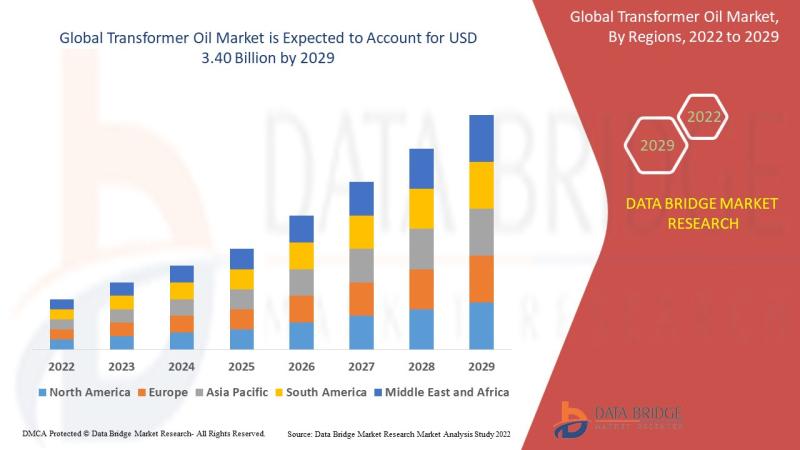
Transformer Oil Market is expected to undergo a CAGR of 7.55% by 2029
Transformer Oil Market Analysis and Size
Transformers are almost everywhere, and every power and distribution transformer is filled with the dielectric insulating fluid, which consists high resistance to electricity and cools the transformer. The "bio based oil" is the highest growing type segment because it has better resistance to fire as compared to other oil over the forecast period. Furthermore, the growth of electric grids in developing economies and the upgradation…
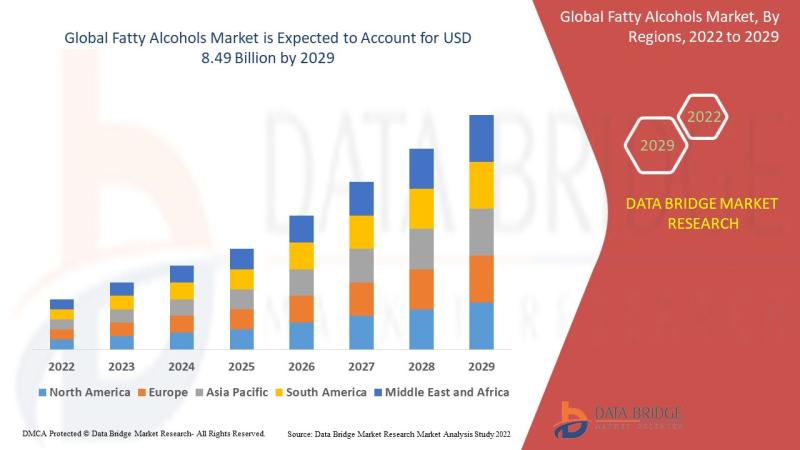
Fatty Alcohols Market is expected to undergo a CAGR of 5.15% by 2029
Fatty Alcohols Market Analysis and Size
The rising demand of hygiene product coupled with growing consumer awareness is anticipated to drive the personal care industry and boost the growth of the fatty alcohols market. Fatty alcohols are used as emollients, emulsifiers and catalytic hydrogenation in cosmetics and beauty products. The "C11-C14 fatty alcohols" is the fastest growing product segment because it is used to produce sodium laureth ether sulphate (SLES), a major foaming agent…
Exclusive Insights on Gusseted Bags Market Latest Trends, Drivers, Strategies an …
An important Gusseted Bags Market research report is produced by taking into account every requirement that organizations need to meet in order to expand successfully. This market report forecasts the market size based on data on major retailer sales, industry growth by upstream and downstream factors, industry advancement, major players, market segments, and application. When creating the reliable Gusseted Bags Market study, the goals of the marketing research are taken into…
More Releases for CTMS
Latest Clinical Trial (CTMS) Market 2022 | Detailed Report
ReportsnReports publishes the report titled Clinical Trial (CTMS) that presents a 360-degree overview of the market under one roof. The report is developed with the meticulous efforts of an enthusiastic and experienced team of experts, analyts, and researchers that makes the report a valuable asset for stakeholders to make robust decisions. This report also provides an in-depth overview of product type, specification, technology, and production analysis considering vital factors like…
Clinical Trial Management System (CTMS) Market commanding maximum share 2025
Clinical Trial Management System (CTMS) Market : Snapshot
The global clinical trial management system market is mainly driven by the heightened occurrences of chronic diseases among people. The rise in the outsourcing of clinical trials is another reason, why this market is expected to flourish in the coming years. Moreover, favorable government initiatives are also anticipated to aid the growth of the global clinical trial management system market. In addition to…
Clinical Trial Management System (CTMS) Market Prophesy In 2025
Clinical Trial Management System (CTMS) Market : Snapshot
The global clinical trial management system market is mainly driven by the heightened occurrences of chronic diseases among people. The rise in the outsourcing of clinical trials is another reason, why this market is expected to flourish in the coming years. Moreover, favorable government initiatives are also anticipated to aid the growth of the global clinical trial management system market. In addition to…
Clinical Trial Management System (CTMS) Market Foreseeable Future by 2025
Clinical Trial Management System (CTMS) Market : Snapshot
The global clinical trial management system market is mainly driven by the heightened occurrences of chronic diseases among people. The rise in the outsourcing of clinical trials is another reason, why this market is expected to flourish in the coming years. Moreover, favorable government initiatives are also anticipated to aid the growth of the global clinical trial management system market. In addition to…
Clinical Trial Management System (CTMS) Market : Estimated to Flourish by 2025
Clinical Trial Management System (CTMS) Market : Snapshot
The global clinical trial management system market is mainly driven by the heightened occurrences of chronic diseases among people. The rise in the outsourcing of clinical trials is another reason, why this market is expected to flourish in the coming years. Moreover, favorable government initiatives are also anticipated to aid the growth of the global clinical trial management system market. In addition to…
Bio-Optronics Announces Advanced Business Intelligence Reporting for CTMS
February 9, 2017 – ROCHESTER, NY – Bio-Optronics, the makers of the market-leading Clinical Conductor clinical trial management system (CTMS) announces the release of an advanced business intelligence (BI) reporting module designed to provide health systems, CROs and other research organizations real-time access to analytics that drive decisions and business growth.
The BI reporting tool, available as a module in Clinical Conductor CTMS, allows users to quickly create recurring and ad-hoc…
