Press release
Structural Heart Devices Market Will Register at a CAGR of 10.2% by 2023| Edwards Lifesciences Corporation, Medtronic plc, Abbott, Boston Scientific Corporation, LivaNova plc
Structural Heart Devices Market research report gives a thorough idea about the current scenario of the global market, recent developments, product launches, joint ventures, capacity, production value, mergers, and acquisitions based on several market dynamics. It gives key measurements, the status of the manufacturers, and is a significant source of direction for the businesses and organizations. Qualitative and transparent research studies are carried out devotedly to offer an outstanding market research report for the niche. Structural Heart Devices Market report potentially endows with numerous insights and business solutions that will assist to stay ahead of the competition.Request for FREE Sample Report: To Know the Impact of COVID-19 on this Industry at: https://www.reportsnreports.com/contacts/requestsample.aspx?name=1646271
This wide-ranging Structural Heart Devices Market research report is sure to help grow the business in several ways. The market insights gained through this market research analysis report facilitates more defined understanding of the market landscape, issues that may interrupt in the future, and ways to position definite brand excellently. The report also includes a range of inhibitors as well as driving forces of the product's market which are analyzed in both qualitative and quantitative approaches so that readers and users get precise information and insights. This Structural Heart Devices Market report has numerous industry-related insights that will provide best solutions and assist to lead the competition.
Structural Heart Devices Market by Product (Heart Valve Devices (Transcatheter and Surgical), Occluders and Delivery Systems, Annuloplasty Rings, and Accessories), Procedure (Replacement and Repair) - Global Forecast to 2023
The major players operating in the structural heart devices market are
Edwards Lifesciences Corporation (US),
Medtronic plc (Ireland),
Abbott (US),
Boston Scientific Corporation (US),
and LivaNova plc (UK).
The rising prevalence of structural heart diseases, regulatory approvals for new and advanced structural heart devices, favorable reimbursement scenario for structural heart procedures & devices, and increasing awareness about structural heart diseases are the major factors driving the growth of the structural heart devices market.
“The replacement proceduressegment is projected to register the highest CAGR during the forecast period.”
Based on procedure, the structural heart devicesmarket is segmentedreplacement procedures and repair procedures. The replacement procedures segment is further segmented into TAVR procedures and SAVR procedures, while the repair procedures segment is subdivided into closure procedures, annuloplasty, valvuloplasty, and TMVR procedures.The replacement procedures segment is expected to register the highest CAGR during the forecast period. This can be attributed to the long-term durability of these procedures and the widespread preference for transcatheter replacement.
“Asia Pacificto witness the highest growth during the forecast period.”
The structural heart devices market in the Asia Pacificis projectedto register the highest CAGRduringthe forecast period. Factors such as the rising geriatric population, favorable reimbursement scenario, increasing regulatory approvals, the presence of a large target patient population, increasing healthcare expenditure, implementation of government-funded insurance schemes, and the growing medical tourism industry in several APAC countries are expected to drive the growth of the Asia Pacific structural heart devices market.
Break of primary participants is mentioned below:
• By CompanyType:Tier 1-62%, Tier 2- 28%, and Tier 3- 10%
• By Designation: C-level - 30%, Director Level - 13%, and Others - 57%
• By Region: North America - 38%, Europe - 20%, Asia Pacific - 30%, and the RoW - 12%,
Research Coverage:
The report analyzes the structural heart devicesmarket and aims at estimating themarket size and the future growth potential of this market based on various segments (such as product, procedure,and region). The report also includes an in-depth regulatory analysis for various regions across the globe and the competitive analysis of the key players in this market along with their company profiles, recent developments, and key strategiesadopted by them to remain competitive in the market.
Reasons to Buy the Report
The report will enrich established firms as well as new entrants/smaller firms to gauge the pulse of the market, which in turn would helpthemgarner a greater share. Firms purchasing the report could use any one or acombination of the below-mentioned strategies.
This report provides insights on the following pointers:
• Market Penetration:Comprehensive information on theproduct portfolios offered by the top players in the global structural heart devicesmarket. The report analyzes the global structural heart devicesmarket, byproduct, procedure, and region.
• Product Enhancement/Innovation: Detailed insights on the upcoming trends and new productlaunches in the global structural heart devicesmarket
• Market Development:Comprehensive information on the lucrative emerging markets, by product, procedure, and region
• Market Diversification:Exhaustive information about new products, growing geographies, recent developments, and investments in the global structural heart devicesmarket
• Competitive Assessment: In-depth assessment of market shares, growth strategies, products, and capabilities of leading players in the global structural heart devicesmarket
Purchase this Structural Heart Devices Market report at: https://www.reportsnreports.com/purchase.aspx?name=1646271
Table of Contents
1 Introduction (Page No. - 15)
1.1 Objectives of the Study
1.2 Market Definition
1.3 Market Scope
1.3.1 Markets Covered
1.3.2 Years Considered for the Study
1.4 Currency
1.5 Limitations
1.6 Stakeholders
2 Research Methodology (Page No. - 18)
2.1 Research Data
2.1.1 Secondary Data
2.1.1.1 Key Data From Secondary Sources
2.1.2 Primary Data
2.1.2.1 Key Data From Primary Sources
2.1.2.2 Key Industry Insights
2.2 Market Size Estimation
2.3 Market Data Estimation and Triangulation
2.4 Assumptions for the Study
3 Executive Summary (Page No. - 27)
4 Premium Insights (Page No. - 30)
4.1 Structural Heart Devices: Market Overview
4.2 Geographic Analysis: Structural Heart Devices Market, By Repair Procedures
4.3 Structural Heart Devices Market, By Procedure, 2018 vs 2023
4.4 Structural Heart Devices Market, By Product, 2018 vs 2023
4.5 Geographic Snapshot of the Structural Heart Devices Market
5 Market Overview (Page No. - 34)
5.1 Introduction
5.2 Market Dynamics
5.2.1 Drivers
5.2.1.1 Rising Prevalence of Structural Heart Diseases
5.2.1.2 Regulatory Approvals of New and Advanced Structural Heart Devices
5.2.1.2.1 Product Approvals for Medtronic
5.2.1.2.2 Product Approvals for Edwards Lifesciences Corporation
5.2.1.2.3 Product Approvals for Abbott Laboratories
5.2.1.2.4 Product Approvals for Livanova
5.2.1.2.5 Product Approvals for Micro Interventional Devices
5.2.1.3 Favorable Reimbursement Scenario
5.2.1.4 Increasing Awareness About Structural Heart Diseases
5.2.2 Restraints
5.2.2.1 Low Affordability and Inaccessibility of Cardiac Surgeries in Developing Countries
5.2.3 Opportunities
5.2.3.1 Percutaneous Interventions, Approval of TAVR for Extended Indications
5.2.3.2 Emerging Countries
5.2.3.3 Emergence of Tissue-Engineered Heart Valves
5.2.4 Challenges
5.2.4.1 Dearth of Skilled Personnel
6 Structural Heart Devices Market, By Product (Page No. - 42)
6.1 Introduction
6.2 Heart Valve Devices
6.2.1 Transcatheter Heart Valves
6.2.2 Surgical Heart Valves
6.2.2.1 Tissue Heart Valves
6.2.2.2 Mechanical Heart Valves
6.3 Occluders & Delivery Systems
6.4 Annuloplasty Rings
6.5 Accessories
6.6 Other Devices
7 Structural Heart Devices Market, By Procedure (Page No. - 52)
7.1 Introduction
7.1.1 Replacement Procedures
7.1.1.1 TAVR Procedures
7.1.1.2 Savr Procedures
7.1.2 Repair Procedures
7.1.2.1 Closure Procedures
7.1.2.2 Annuloplasty
7.1.2.3 Valvuloplasty
7.1.2.4 TMVR Procedures
8 Structural Heart Devices Market, By Region (Page No. - 63)
8.1 Introduction
8.2 North America
8.2.1 US
8.2.2 Canada
8.3 Europe
8.3.1 Germany
8.3.2 Italy
8.3.3 UK
8.3.4 France
8.3.5 Rest of Europe
8.4 Asia Pacific
8.4.1 Japan
8.4.2 China
8.4.3 India
8.4.4 Rest of Asia Pacific
8.5 Rest of the World
9 Competitive Landscape (Page No. - 108)
9.1 Overview
9.2 Market Ranking Analysis of Key Players, 2017
9.3 Competitive Scenario
9.3.1 Product Launches and Approvals
9.3.2 Acquisitions
9.3.3 Expansions
9.3.4 Partnerships and Agreements
10 Company Profiles (Page No. - 113)
(Introduction, Products & Services, Strategy, & Analyst Insights, Developments, MnM View)*
10.1 Edwards Lifesciences Corporation
10.2 Medtronic
10.3 Abbott
10.4 Boston Scientific Corporation
10.5 Livanova
10.6 Lepu Medical Technology
10.7 Cryolife
10.8 Micro Interventional Devices
10.9 Braile Biomédica
10.10 TTK Healthcare (A TTK Group Company)
Enquire Here for, Report Enquiry, Discount and Customization at: https://www.reportsnreports.com/contacts/discount.aspx?name=1646271
Contact Us:
Corporate Headquarters
Tower B5, office 101,
Magarpatta SEZ,
Hadapsar, Pune-411013, India
+ 1 888 391 5441
sales@reportsandreports.com
About Us:
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Structural Heart Devices Market Will Register at a CAGR of 10.2% by 2023| Edwards Lifesciences Corporation, Medtronic plc, Abbott, Boston Scientific Corporation, LivaNova plc here
News-ID: 2534642 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
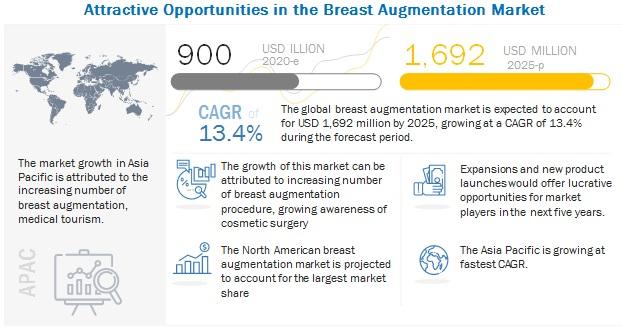
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Heart
Heart disease and Heart Attacks - Kaffailham.gr
Καρδιολόγος Αθηνα - https://kaffailham.gr
When people talk about heart disease or heart attacks. The arteries that they are talking about are the arteries that actually provide blood to the heart. Remember the heart itself is a muscle. It itself needs oxygen. So you have these arteries right over here, the red tubes. Those are arteries. and then the blue ones are veins. They're taking the de-oxygenated blood away from the tissue…
Artificial Heart Implant Market May See a Big Move | Major Giants Jarvik Heart, …
The Latest survey report on Artificial Heart Implant Market sheds lights on changing dynamics of each of the subsegments of Industry. As the shift to value continues, Artificial Heart Implant organizations have the dual challenge of increasing interoperability to improve clinical performance and the patient experience. Some of the companies listed in the study from complete survey list are Medtronic, Boston Scientific, Abbott, Terumo, B.Braun, SynCardia, BiVACOR, CARMAT, ReinHeart TAH…
Heart Pump Device Market - Potential Innovations 2025 | Terumo, Jarvik Heart
Heart Pump Device Market: Snapshot
Heart pump devices are essentially mechanical pumps playing the role of ventricular assist devices. These are surgically implanted and are used for temporarily supporting the functions of heart in people with weak heart or irregular blood flow. These devices are notably used as a bridge to cardiac transplantation for patients suffering with end stage heart failure. They can also be used in patients during and after…
Congestive Heart Failure (Heart Failure)-Pipeline Review, H2 2017
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Congestive Heart Failure (Heart Failure)-Pipeline Review, H2 2017, provides an overview of the Congestive Heart Failure (Heart Failure) (Cardiovascular) pipeline landscape.
Heart failure is also known as congestive heart failure (CHF). CHF is a condition in which the heart is no longer able to pump out enough oxygen-rich blood. Symptoms include cough, fatigue, weakness, faintness, loss of appetite, swollen (enlarged) liver…
Congestive Heart Failure (Heart Failure) - Pipeline Review, H2 2017
ReportsWorldwide has announced the addition of a new report title Congestive Heart Failure (Heart Failure) - Pipeline Review, H2 2017 to its growing collection of premium market research reports.
Summary
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Congestive Heart Failure (Heart Failure) - Pipeline Review, H2 2017, provides an overview of the Congestive Heart Failure (Heart Failure) (Cardiovascular) pipeline landscape.
Heart failure is also known as congestive heart failure (CHF).…
Congestive Heart Failure (Heart Failure) Market Research Report
Latest industry research report on: Congestive Heart Failure (Heart Failure) Market | Industry Size, Share, Research, Reviews, Analysis, Strategies, Demand, Growth, Segmentation, Parameters, Forecasts
GlobalData's clinical trial report, Congestive Heart Failure (Heart Failure) Global Clinical Trials Review, H1, 2017" provides an overview of Congestive Heart Failure (Heart Failure) clinical trials scenario. This report provides top line data relating to the clinical trials on Congestive Heart Failure (Heart Failure). Report includes an…